A Novel Mycovirus Evokes Transcriptional Rewiring in the Fungus Malassezia and Stimulates Beta Interferon Production in Macrophages
- PMID: 32873760
- PMCID: PMC7468202
- DOI: 10.1128/mBio.01534-20
A Novel Mycovirus Evokes Transcriptional Rewiring in the Fungus Malassezia and Stimulates Beta Interferon Production in Macrophages
Abstract
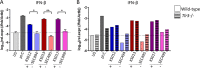
Mycoviruses infect fungi, and while most persist asymptomatically, there are examples of mycoviruses having both beneficial and detrimental effects on their host. Virus-infected Saccharomyces and Ustilago strains exhibit a killer phenotype conferring a growth advantage over uninfected strains and other competing yeast species, whereas hypovirus-infected Cryphonectria parasitica displays defects in growth, sporulation, and virulence. In this study, we identify a double-stranded RNA (dsRNA) mycovirus in five Malassezia species. Sequence analysis reveals it to be a totivirus with two dsRNA segments: a larger 4.5-kb segment with genes encoding components for viral replication and maintenance, and a smaller 1.4-kb segment encoding a novel protein. Furthermore, transcriptome sequencing (RNA-seq) of virus-infected versus virus-cured Malassezia sympodialis revealed an upregulation of dozens of ribosomal components in the cell, suggesting the virus modifies the transcriptional and translational landscapes of the cell. Given that Malassezia is the most abundant fungus on human skin, we assessed the impact of the mycovirus in a murine epicutaneous infection model. Although infection with virus-infected strains was not associated with an increased inflammatory response, we did observe enhanced skin colonization in one of two virus-infected M. sympodialis strains. Noteworthy, beta interferon expression was significantly upregulated in bone marrow-derived macrophages when challenged with virus-infected, compared to virus-cured, M. sympodialis, suggesting that the presence of the virus can induce an immunological response. Although many recent studies have illuminated how widespread mycoviruses are, there are relatively few in-depth studies about their impact on disease caused by the host fungus. We describe here a novel mycovirus in Malassezia and its possible implications in pathogenicity.IMPORTANCEMalassezia species represent the most common fungal inhabitant of the mammalian skin microbiome and are natural skin commensal flora. However, these fungi are also associated with a variety of clinical skin disorders. Recent studies have reported associations of Malassezia with Crohn's disease and pancreatic cancer, further implicating this fungal genus in inflammatory and neoplastic disease states. Because M. sympodialis has lost genes involved in RNA interference (RNAi), we hypothesized Malassezia could harbor dsRNA mycoviruses. Indeed, we identified a novel mycovirus of the totivirus family in several Malassezia species and characterized the MsMV1 mycovirus of M. sympodialis We found conditions that lead to curing of the virus and analyzed isogenic virus-infected/virus-cured strains to determine MsMV1 genetic and pathogenic impacts. MsMV1 induces a strong overexpression of transcription factors and ribosomal genes, while downregulating cellular metabolism. Moreover, MsMV1 induced a significantly higher level of beta interferon expression in cultured macrophages. This study sheds light on the mechanisms of pathogenicity of Malassezia, focusing on a previously unidentified novel mycovirus.
Keywords: Malassezia; beta interferon; mycoviruses; skin.
Copyright © 2020 Applen Clancey et al.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
