Adsorption of rare earth elements in regolith-hosted clay deposits
- PMID: 32873784
- PMCID: PMC7463018
- DOI: 10.1038/s41467-020-17801-5
Adsorption of rare earth elements in regolith-hosted clay deposits
Abstract
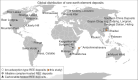
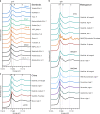
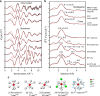
Global resources of heavy Rare Earth Elements (REE) are dominantly sourced from Chinese regolith-hosted ion-adsorption deposits in which the REE are inferred to be weakly adsorbed onto clay minerals. Similar deposits elsewhere might provide alternative supply for these high-tech metals, but the adsorption mechanisms remain unclear and the adsorbed state of REE to clays has never been demonstrated in situ. This study compares the mineralogy and speciation of REE in economic weathering profiles from China to prospective regoliths developed on peralkaline rocks from Madagascar. We use synchrotron X-ray absorption spectroscopy to study the distribution and local bonding environment of Y and Nd, as proxies for heavy and light REE, in the deposits. Our results show that REE are truly adsorbed as easily leachable 8- to 9-coordinated outer-sphere hydrated complexes, dominantly onto kaolinite. Hence, at the atomic level, the Malagasy clays are genuine mineralogical analogues to those currently exploited in China.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Weng Z, Jowitt SM, Mudd GM, Haque N. A detailed assessment of global rare earth element resources: opportunities and challenges. Econ. Geol. 2015;110:1925–1952.
-
- Jowitt SM, Wong VNL, Wilson SA, Gore O. Critical metals in the critical zone: controls, resources and future prospectivity of regolith-hosted rare earth elements AU. Aust. J. Earth Sci. 2017;64:1045–1054.
-
- Goodenough, K. M., Wall, F. & Merriman, D. The rare earth elements: demand, global resources, and challenges for resourcing future generations. Nat. Resour. Res. 10.1007/s11053-017-9336-5, 1–16 (2017).
-
- Ober, J. A. Mineral Commodity Summaries 2018 (US Geological Survey, 2018).
-
- European Commission. Study on the review of the list of Critical Raw Materials—Final Report (European Union, 2017).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

