Precise and error-prone CRISPR-directed gene editing activity in human CD34+ cells varies widely among patient samples
- PMID: 32873924
- PMCID: PMC7902267
- DOI: 10.1038/s41434-020-00192-z
Precise and error-prone CRISPR-directed gene editing activity in human CD34+ cells varies widely among patient samples
Abstract
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and their associated CRISPR-associated nucleases (Cas) are among the most promising technologies for the treatment of hemoglobinopathies including Sickle Cell Disease (SCD). We are only beginning to identify the molecular variables that influence the specificity and the efficiency of CRISPR- directed gene editing, including the position of the cleavage site and the inherent variability among patient samples selected for CRISPR-directed gene editing. Here, we target the beta globin gene in human CD34+ cells to assess the impact of these two variables and find that both contribute to the global diversity of genetic outcomes. Our study demonstrates a unique genetic profile of indels that is generated based on where along the beta globin gene attempts are made to correct the SCD single base mutation. Interestingly, even within the same patient sample, the location of where along the beta globin gene the DNA is cut, HDR activity varies widely. Our data establish a framework upon which realistic protocols inform strategies for gene editing for SCD overcoming the practical hurdles that often impede clinical success.
Trial registration: ClinicalTrials.gov NCT03745287.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

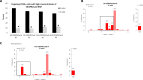


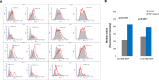
References
-
- Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014:346. 10.1126/science.1258096. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

