Spontaneous mirror symmetry breaking in benzil-based soft crystalline, cubic liquid crystalline and isotropic liquid phases
- PMID: 32874512
- PMCID: PMC7446726
- DOI: 10.1039/d0sc01396j
Spontaneous mirror symmetry breaking in benzil-based soft crystalline, cubic liquid crystalline and isotropic liquid phases
Abstract
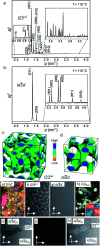
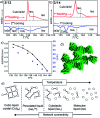
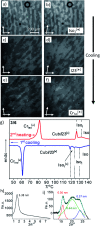
Benzil (diphenylethane-1,2-dione), which is a long known example for an achiral molecule crystallizing in a chiral space group, can also show mirror symmetry breaking in the fluid state if it is suitably functionalized. For some of the new benzil derivatives even three different subsequent mirror symmetry broken soft matter states with a chiral conglomerate structure can be observed. One is an isotropic liquid, the second one a cubic liquid crystal with a complex network structure and the third is a soft crystalline solid. Chirality develops by helical self-assembly combined with dynamic network formation, thus allowing macroscopic chirality synchronization. These achiral molecules, combining a transiently chiral bent core with multiple alkyl chains, provide a unique link between the mirror symmetry breaking phenomena observed for polycatenar and bent-core mesogens. The homogeneously chiral networks are of interest for application as chiral materials, and as templates for chiral recognition, separation and enantioselective catalysis.
This journal is © The Royal Society of Chemistry 2020.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Guijarro A. and Yus M., The Origin of Chirality in the Molecules of Life, RSC, Publishing, Cambridge, 2009
-
- Amabilino D. B. Kellogg R. M. Isr. J. Chem. 2011;51:1034–1040. doi: 10.1002/ijch.201100051. - DOI
-
- Viedma C. Phys. Rev. Lett. 2005;94:065504. doi: 10.1103/PhysRevLett.94.065504. - DOI - PubMed
- Cintas P. Viedma C. Chirality. 2012;24:894–898. doi: 10.1103/PhysRevLett.94.065504. - DOI - PubMed
- Hein J. E. Cao B. H. Viedma C. Kellogg R. M. Blackmond D. G. J. Am. Chem. Soc. 2012;134:12629–12636. doi: 10.1103/PhysRevLett.94.065504. - DOI - PubMed
-
- Steendam R. R. E. Verkade J. M. M. van Benthem T. J. B. Meekes H. van Enckevort W. J. P. Raap J. Rutjes F. P. J. T. Vlieg E. Nat. Commun. 2014;5:5543. doi: 10.1038/ncomms6543. - DOI - PMC - PubMed
- Uemura N. Sano K. Matsumoto A. Yoshida Y. Mino T. Sakamoto M. Chem.–Asian J. 2019;14:4150–4153. doi: 10.1038/ncomms6543. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

