Luminescent tungsten(vi) complexes as photocatalysts for light-driven C-C and C-B bond formation reactions
- PMID: 32874518
- PMCID: PMC7448528
- DOI: 10.1039/d0sc01340d
Luminescent tungsten(vi) complexes as photocatalysts for light-driven C-C and C-B bond formation reactions
Abstract
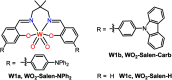
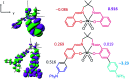
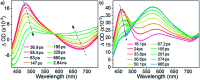
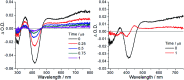
The realization of photocatalysis for practical synthetic application hinges on the development of inexpensive photocatalysts which can be prepared on a large scale. Herein an air-stable, visible-light-absorbing photoluminescent tungsten(vi) complex which can be conveniently prepared at the gram-scale is described. This complex could catalyse photochemical organic transformation reactions including borylation of aryl halides, such as aryl chloride, reductive coupling of benzyl bromides for C-C bond formation, reductive coupling of phenacyl bromides, and decarboxylative coupling of redox-active esters of alkyl carboxylic acid with high product yields and broad functional group tolerance.
This journal is © The Royal Society of Chemistry 2020.
Figures




References
-
- Shaw M. H., Twilton J., MacMillan D. W. C. J. Org. Chem. 2016;81:6898. - PMC - PubMed
- Liu Q., Wu L.-Z. Natl. Sci. Rev. 2017;4:359.
- Parasram M., Gevorgyan V. Chem. Soc. Rev. 2017;46:6227. - PMC - PubMed
- Twilton J., Le C., Zhang P., Shaw M. H., Evans R. W., MacMillan D. W. C. Nat. Rev. Chem. 2017;1:0052.
- Fensterbank L., Goddard J.-P. and Ollivier C., in Visible Light Photocatalysis in Organic Chemistry, ed. C. Stephenson, T. Yoon and D. W. C. MacMillan, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim, 2018.
- Larsen C. B., Wenger O. S. Chem. – Eur. J. 2018;24:2039. - PubMed
- Marzo L., Pagire S. K., Reiser O., Konig B. Angew. Chem., Int. Ed. 2018;57:10034. - PubMed
- McAtee R. C., McClain E. J., Stephenson C. R. J. Trends Chem. 2019;1:111. - PMC - PubMed
- Glaser F., Wenger O. S. Coord. Chem. Rev. 2020;405:213129.
- Hockin B. M., Li C., Robertson N., Zysman-Colman E. Catal. Sci. Technol. 2019;9:889.
- Förster C., Heinze K. Chem. Soc. Rev. 2020;49:1057. - PubMed
-
- Dietrich-Buchecker C. O., Marnot P. A., Sauvage J.-P., Kirchhoff J. R., McMillin D. R. J. Chem. Soc., Chem. Commun. 1983:513.
- Kern J.-M., Sauvage J.-P. J. Chem. Soc., Chem. Commun. 1987:546.
- Li D., Che C.-M., Kwong H.-L., Yam V. W.-W. J. Chem. Soc., Dalton Trans. 1992:3325.
- Cuttell D. G., Kuang S.-M., Fanwick P. E., McMillin D. R., Walton R. A. J. Am. Chem. Soc. 2002;124:6. - PubMed
- Wang B., Shelar D. P., Han X.-Z., Li T.-T., Guan X., Lu W., Liu K., Chen Y., Fu W.-F., Che C.-M. Chem. – Eur. J. 2015;21:1184. - PubMed
- Knorn M., Rawner T., Czerwieniec R., Reiser O. ACS Catal. 2015;5:5186.
- Cetin M. M., Hodson R. T., Hart C. R., Cordes D. B., Findlater M., Casadonte Jr D. J., Cozzolino A. F., Mayer M. F. Dalton Trans. 2017;46:6553. - PubMed
-
- Grubel M., Bosque I., Altmann P. J., Bach T., Hess C. R. Chem. Sci. 2018;9:3313. - PMC - PubMed
- Buldt L. A., Larsen C. B., Wenger O. S. Chem. – Eur. J. 2017;23:8577. - PubMed
- Malzkuhn S., Wenger O. S. Coord. Chem. Rev. 2018;359:52.
- Lim C.-H., Kudisch M., Liu B., Miyake G. M. J. Am. Chem. Soc. 2018;140:7667. - PMC - PubMed
- Shen X., Li Y., Wen Z., Cao S., Hou X., Gong L. Chem. Sci. 2018;9:4562. - PMC - PubMed
- Shields B. J., Kudisch B., Scholes G. D., Doyle A. G. J. Am. Chem. Soc. 2018;140:3035. - PMC - PubMed
- Frem R. C. G., Massabni A. C., Massabni A. M. G., Mauro A. E. Inorg. Chim. Acta. 1997;255:53.
- Kunkely H., Vogler A. Inorg. Chem. Commun. 2000;3:143.
LinkOut - more resources
Full Text Sources

