Methylbismuth: an organometallic bismuthinidene biradical
- PMID: 32874526
- PMCID: PMC7450715
- DOI: 10.1039/d0sc02410d
Methylbismuth: an organometallic bismuthinidene biradical
Abstract
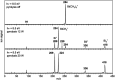
We report the generation, spectroscopic characterization, and computational analysis of the first free (non-stabilized) organometallic bismuthinidene, BiMe. The title compound was generated in situ from BiMe3 by controlled homolytic Bi-C bond cleavage in the gas phase. Its electronic structure was characterized by a combination of photoion mass-selected threshold photoelectron spectroscopy and DFT as well as multi-reference computations. A triplet ground state was identified and an ionization energy (IE) of 7.88 eV was experimentally determined. Methyl abstraction from BiMe3 to give [BiMe2]• is a key step in the generation of BiMe. We reaveal a bond dissociation energy of 210 ± 7 kJ mol-1, which is substantially higher than the previously accepted value. Nevertheless, the homolytic cleavage of Me-BiMe2 bonds could be achieved at moderate temperatures (60-120 °C) in the condensed phase, suggesting that [BiMe2]• and BiMe are accessible as reactive intermediates under these conditions.
This journal is © The Royal Society of Chemistry 2020.
Figures






References
-
- Dostál L. Coord. Chem. Rev. 2017;353:142–158.
-
- Dielmann F., Back O., Henry-Ellinger M., Jerabek P., Frenking G., Bertrand G. Science. 2012;337:1526–1528. - PubMed
-
- Liu L., Ruiz D. A., Munz D., Bertrand G. Chem. 2016;1:147–153.
-
- Šimon P., de Proft F., Jambor R., RůŽička A., Dostál L. Angew. Chem., Int. Ed. 2010;49:5468–5471. - PubMed
-
- Vranova I., Alonso M., Lo R., Sedlak R., Jambor R., Ruzicka A., De Proft F., Hobza P., Dostál L. Chem.–Eur. J. 2015;21:16917–16928. - PubMed
LinkOut - more resources
Full Text Sources

