Resident macrophages as potential therapeutic targets for cardiac ageing and injury
- PMID: 32874584
- PMCID: PMC7450172
- DOI: 10.1002/cti2.1167
Resident macrophages as potential therapeutic targets for cardiac ageing and injury
Abstract
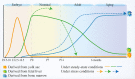
Cardiac-resident macrophages (CRMs) play critical roles in maintaining cardiac homoeostasis and removing senescent and dying cells. Recent preclinical data have re-energised the area of cardioimmunology and provided improved understanding of the modulation of compositional and functional phenotypes of CRMs. These data can aid in achieving improved cardiac regeneration, repair and functional remodelling following cardiac injury. In this review, we discuss the composition and renewal of various subsets of CRMs. Specific attention has been given to delineate the roles of various CRM subsets with respect to (1) facilitation of cardiac development and maintenance of physiological function such as electrical conduction and rhythm; (2) promotion of cardiac regeneration, inflammation resolution and functional remodelling following a cardiac injury; and (3) therapeutic potential. We have also highlighted the relationship between CRM replenishment and cardiomyocyte senescence as well as cardiovascular diseases development. Finally, we have addressed future perspectives and directions in basic research and potentially clinical applications of CRMs.
Keywords: cardiac development; cardiac injury; cardiac regeneration; cardiac‐resident macrophages; cardiomyocyte senescence.
© 2020 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
