Thalidomide for refractory gastrointestinal bleeding from vascular malformations in patients with significant comorbidities
- PMID: 32874976
- PMCID: PMC7441260
- DOI: 10.12998/wjcc.v8.i15.3218
Thalidomide for refractory gastrointestinal bleeding from vascular malformations in patients with significant comorbidities
Abstract
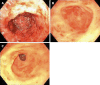
Background: Refractory gastrointestinal bleeding (GIB) secondary to gastrointestinal vascular malformations (GIVM) such as gastrointestinal angiodysplasia (GIAD) and gastric antral vascular ectasia (GAVE) remains challenging to treat when endoscopic therapy fails. Recently thalidomide has been suggested as a treatment option for refractory GIB.
Aim: To determine the outcome of patients treated with thalidomide for refractory GIB due to GIVM.
Methods: IRB approved, single center, retrospective review of electronic medical records from January 2012 to November 2018. Patients age > 18 years old, who had > 3 episodes of GIB refractory to medical or endoscopic therapy, and who had been treated with thalidomide for at least 3 mo were included. The primary endpoint was recurrence of GIB 6 mo after initiation of thalidomide.
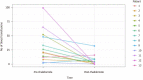
Results: Fifteen patients were included in the study, all with significant cardiac, hepatic, or renal comorbidities. The cause of GIB was GIAD in 10 patients and GAVE in 5 patients. Two patients were lost to follow up. Of the 13 patients followed, 38.5% (n = 5) had no recurrent GIB or transfusion requirement after treatment with thalidomide. Furthermore, 84.6% (n = 11) of patients had a reduction in transfusion requirements and hospitalizations for GIB. Thalidomide was discontinued in 2 patients due to cost (n = 1) and medication interaction (n = 1). Reported adverse reactions included fatigue (n = 3), neuropathy (n = 2), dizziness (n = 1), and constipation (n = 1). Six patients died during follow up due to unknown cause (n = 4) and sepsis (n = 2).
Conclusion: Thalidomide appears to be an effective treatment for refractory GIB due to GIAD or GAVE in a Western population with significant comorbidities.
Keywords: Angiodysplasia; Gastric antral vascular ectasia; Refractory gastrointestinal bleeding; Thalidomide; Vascular malformation.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Both authors have no conflicts of interest.
Figures
References
-
- Junquera F, Saperas E, de Torres I, Vidal MT, Malagelada JR. Increased expression of angiogenic factors in human colonic angiodysplasia. Am J Gastroenterol. 1999;94:1070–1076. - PubMed
-
- Nardone G, Compare D, Martino A, Rocco A. Pharmacological treatment of gastrointestinal bleeding due to angiodysplasias: A position paper of the Italian Society of Gastroenterology (SIGE) Dig Liver Dis. 2018;50:542–548. - PubMed
-
- Draper KV, Huang RJ, Gerson LB. GI bleeding in patients with continuous-flow left ventricular assist devices: a systematic review and meta-analysis. Gastrointest Endosc. 2014;80:435–446.e1. - PubMed
-
- Chalasani N, Cotsonis G, Wilcox CM. Upper gastrointestinal bleeding in patients with chronic renal failure: role of vascular ectasia. Am J Gastroenterol. 1996;91:2329–2332. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

