Clinical and Patient-Reported Outcomes of Direct-Acting Antivirals for the Treatment of Chronic Hepatitis C Among Patients on Opioid Agonist Treatment: A Real-world Prospective Cohort Study
- PMID: 32875003
- PMCID: PMC7452367
- DOI: 10.1093/ofid/ofaa317
Clinical and Patient-Reported Outcomes of Direct-Acting Antivirals for the Treatment of Chronic Hepatitis C Among Patients on Opioid Agonist Treatment: A Real-world Prospective Cohort Study
Abstract
Background: Patient-reported outcomes (PROs) can help to reduce uncertainties about hepatitis C virus (HCV) treatment with direct-acting antivirals (DAAs) among people who inject drugs and increase treatment uptake in this high-risk group. Besides clinical data, this study analyzed for the first time PROs in a real-world sample of patients on opioid agonist treatment (OAT) and HCV treatment with DAAs.
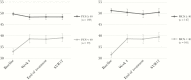
Methods: HCV treatment data including virological response, adherence, safety, and PROs of 328 German patients on OAT were analyzed in a pragmatic prospective cohort study conducted from 2016 to 2018. Clinical effectiveness was defined as sustained virological response (SVR) at week 12 after end of treatment and calculated in per-protocol (PP) and intention-to-treat (ITT) analyses. Changes over time in PROs on health-related quality of life, physical and mental health, functioning, medication tolerability, fatigue, concentration, and memory were analyzed by repeated-measures analyses of variances (ANOVAs).
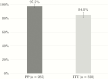
Results: We found high adherence and treatment completion rates, a low number of mainly mild adverse events, and high SVR rates (PP: 97.5% [n = 285]; ITT: 84.5% [n = 328]). Missing SVR data in the ITT sample were mainly caused by patients lost to follow-up after treatment completion. Most PROs showed statistically significant but modest improvements over time, with more pronounced improvements in highly impaired patients.
Conclusions: This real-world study confirms that DAA treatment among OAT patients is feasible, safe, and effective. PROs show that all patients, but particularly those with higher somatic, mental, and social burden, benefit from DAA treatment.
Keywords: direct-acting antivirals; hepatitis C virus; opioid substitution treatment; patient-reported outcome measures.
© The Author(s) 2020. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures

References
-
- Schulte B, Schmidt CS, Strada L, et al. . Hepatitis C virus prevalence and incidence in a large nationwide sample of patients in opioid substitution treatment in Germany: a prospective cohort study. Clin Infect Dis 2020; 10:2199–205. - PubMed
-
- Macias J, Morano LE, Tellez F, et al. . Response to direct-acting antiviral therapy among ongoing drug users and people receiving opioid substitution therapy. J Hepatol 2019; 71:45–51. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

