Long-term safety of vedolizumab for inflammatory bowel disease
- PMID: 32876349
- PMCID: PMC7540482
- DOI: 10.1111/apt.16060
Long-term safety of vedolizumab for inflammatory bowel disease
Abstract
Background: Vedolizumab, a gut-selective α4 β7 integrin antibody, is approved for moderately to severely active ulcerative colitis (UC) and Crohn's disease (CD).
Aim: To report the final results from the vedolizumab GEMINI long-term safety (LTS) study.
Methods: The phase 3, open-label GEMINI LTS study (initiated May 2009) enrolled patients with UC or CD from four prior clinical trials and vedolizumab-naïve patients. Vedolizumab LTS was evaluated; efficacy and patient-reported outcomes were exploratory endpoints.
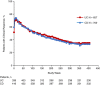
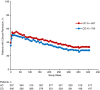
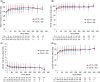
Results: Enrolled patients (UC, n = 894; CD, n = 1349) received vedolizumab 300 mg IV every 4 weeks; median cumulative exposure was 42.4 months (range: 0.03-112.2) for UC and 31.5 months (range: 0.03-100.3) for CD. Over 8 years, adverse events (AEs) occurred in 93% (UC) and 96% (CD) of patients, with UC (36%) and CD (35%) exacerbations most frequent. Serious AEs were reported for 31% (UC) and 41% (CD) of patients. Vedolizumab discontinuation due to AEs occurred in 15% (UC) and 17% (CD) of patients. There were no new trends for infections, malignancies, infusion-related reactions, or hepatic events, and no cases of progressive multifocal leukoencephalopathy. Of the ten deaths (UC, n = 4; CD, n = 6), two were considered drug-related by local investigators (West Nile virus infection-related encephalitis and hepatocellular carcinoma). Continuous vedolizumab maintained clinical response long-term, with 33% (UC) and 28% (CD) of patients in clinical remission at 400 treatment weeks.
Conclusions: The safety profile of vedolizumab remains favourable with no unexpected or new safety concerns. These results further establish the safety of vedolizumab and support its long-term use (NCT00790933/EudraCT 2008-002784-14).
© 2020 Takeda Pharmaceuticals USA. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures

References
-
- Torres J, Mehandru S, Colombel J‐F, Peyrin‐Biroulet L. Crohn's disease. Lancet. 2017;389:1741‐1755. - PubMed
-
- ENTYVIO (vedolizumab) prescribing information. Takeda Pharma/S. Revised 04/01/2019.
-
- Vedolizumab [summary of product characteristics]. Taastrup, Denmark: Takeda Pharma A/S; Revised 04/01/2019.
-
- Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699‐710. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
