CD163 and pAPN double-knockout pigs are resistant to PRRSV and TGEV and exhibit decreased susceptibility to PDCoV while maintaining normal production performance
- PMID: 32876563
- PMCID: PMC7467724
- DOI: 10.7554/eLife.57132
CD163 and pAPN double-knockout pigs are resistant to PRRSV and TGEV and exhibit decreased susceptibility to PDCoV while maintaining normal production performance
Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) and transmissible gastroenteritis virus (TGEV) are two highly infectious and lethal viruses causing major economic losses to pig production. Here, we report generation of double-gene-knockout (DKO) pigs harboring edited knockout alleles for known receptor proteins CD163 and pAPN and show that DKO pigs are completely resistant to genotype 2 PRRSV and TGEV. We found no differences in meat-production or reproductive-performance traits between wild-type and DKO pigs, but detected increased iron in DKO muscle. Additional infection challenge experiments showed that DKO pigs exhibited decreased susceptibility to porcine deltacoronavirus (PDCoV), thus offering unprecedented in vivo evidence of pAPN as one of PDCoV receptors. Beyond showing that multiple gene edits can be combined in a livestock animal to achieve simultaneous resistance to two major viruses, our study introduces a valuable model for investigating infection mechanisms of porcine pathogenic viruses that exploit pAPN or CD163 for entry.
Keywords: CRISPR/Cas9; PDCoV; PRRSV; TGEV; infectious disease; microbiology; pig; virus.
Plain language summary
Pig epidemics are the biggest threat to the pork industry. In 2019 alone, hundreds of billions of dollars worldwide were lost due to various pig diseases, many of them caused by viruses. The porcine reproductive and respiratory virus (PRRS virus for short), for instance, leads to reproductive disorders such as stillbirths and premature labor. Two coronaviruses – the transmissible gastroenteritis virus (or TGEV) and the porcine delta coronavirus – cause deadly diarrhea and could potentially cross over into humans. Unfortunately, there are still no safe and effective methods to prevent or control these pig illnesses, but growing disease-resistant pigs could reduce both financial and animal losses. Traditionally, breeding pigs to have a particular trait is a slow process that can take many years. But with gene editing technology, it is possible to change or remove specific genes in a single generation of animals. When viruses infect a host, they use certain proteins on the surface of the host’s cells to find their inside: the PRRS virus relies a protein called CD163, and TGEV uses pAPN. Xu, Zhou, Mu et al. used gene editing technology to delete the genes that encode the CD163 and pAPN proteins in pigs. When the animals were infected with PRRS virus or TGEV, the non-edited pigs got sick but the gene-edited animals remained healthy. Unexpectedly, pigs without CD163 and pAPN also coped better with porcine delta coronavirus infections, suggesting that CD163 and pAPN may also help this coronavirus infect cells. Finally, the gene-edited pigs reproduced and produced meat as well as the control pigs. These experiments show that gene editing can be a powerful technology for producing animals with desirable traits. The gene-edited pigs also provide new knowledge about how porcine viruses infect pigs, and may offer a starting point to breed disease-resistant animals on a larger scale.
© 2020, Xu et al.
Conflict of interest statement
KX, YZ, YM, ZL, SH, YX, LF, YW, XZ, CX, JC, ZF, GX, JG, HL, SX, JL, KL No competing interests declared, CG is affiliated with Shandong Landsee Genetics Co., Ltd. The author has no financial interests to declare. HS is affiliated with Shenzhen Kingsino Technology Co., Ltd. The author has no financial interests to declare.
Figures
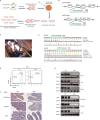

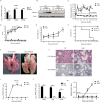
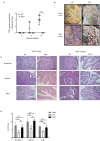
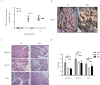


References
-
- Burkard C, Lillico SG, Reid E, Jackson B, Mileham AJ, Ait-Ali T, Whitelaw CB, Archibald AL. Precision engineering for PRRSV resistance in pigs: macrophages from genome edited pigs lacking CD163 SRCR5 domain are fully resistant to both PRRSV genotypes while maintaining biological function. PLOS Pathogens. 2017;13:e1006206. doi: 10.1371/journal.ppat.1006206. - DOI - PMC - PubMed
-
- Chen J, Wang H, Bai J, Liu W, Liu X, Yu D, Feng T, Sun Z, Zhang L, Ma L, Hu Y, Zou Y, Tan T, Zhong J, Hu M, Bai X, Pan D, Xing Y, Zhao Y, Tian K, Hu X, Li N. Generation of pigs resistant to highly Pathogenic-Porcine reproductive and respiratory syndrome virus through gene editing of CD163. International Journal of Biological Sciences. 2019;15:481–492. doi: 10.7150/ijbs.25862. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2016ZX08010-004/National Transgenic Breeding Project/International
- Major Scientific Research Tasks for Scientific and Technological Innovation Project CAAS-ZDRW202006/Chinese Academy of Agricultural Sciences/International
- Kh17134/Shandong Landsee Genetics Co., Ltd./International
- U1704231/National Natural Science Foundation of China/International
- JSGG20180507182028625/Shenzhen Key Technology Projects/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

