Bacteriocins as a new generation of antimicrobials: toxicity aspects and regulations
- PMID: 32876664
- PMCID: PMC7794045
- DOI: 10.1093/femsre/fuaa039
Bacteriocins as a new generation of antimicrobials: toxicity aspects and regulations
Abstract
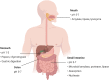
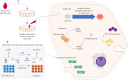
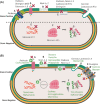
In recent decades, bacteriocins have received substantial attention as antimicrobial compounds. Although bacteriocins have been predominantly exploited as food preservatives, they are now receiving increased attention as potential clinical antimicrobials and as possible immune-modulating agents. Infections caused by antibiotic-resistant bacteria have been declared as a global threat to public health. Bacteriocins represent a potential solution to this worldwide threat due to their broad- or narrow-spectrum activity against antibiotic-resistant bacteria. Notably, despite their role in food safety as natural alternatives to chemical preservatives, nisin remains the only bacteriocin legally approved by regulatory agencies as a food preservative. Moreover, insufficient data on the safety and toxicity of bacteriocins represent a barrier against the more widespread use of bacteriocins by the food and medical industry. Here, we focus on the most recent trends relating to the application of bacteriocins, their toxicity and impacts.
Keywords: antimicrobials; bacteriocins; gastrointestinal bioavailability; regulations; safety evaluation; toxicity.
© The Author(s) 2020. Published by Oxford University Press on behalf of FEMS.
Figures

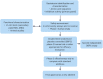
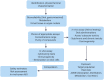
References
-
- Abbasiliasi S, Tan JS, Ibrahim TAT et al. Fermentation factors influencing the production of bacteriocins by lactic acid bacteria: a review. RSC Adv. 2017;7:29395–420.
-
- Ahmad V, Khan MS, Jamal QMS et al. Antimicrobial potential of bacteriocins: in therapy, agriculture and food preservation. Int J Antimicrob Agents. 2017;49:1–11. - PubMed
-
- Akhila JS, Shyamjith D, Alwar M. Acute toxicity studies and determination of median lethal dose. Curr Sci. 2007;93;917–20.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

