Efficacy and Safety of Baricitinib in Chinese Rheumatoid Arthritis Patients and the Subgroup Analyses: Results from Study RA-BALANCE
- PMID: 32876903
- PMCID: PMC7695798
- DOI: 10.1007/s40744-020-00231-6
Efficacy and Safety of Baricitinib in Chinese Rheumatoid Arthritis Patients and the Subgroup Analyses: Results from Study RA-BALANCE
Abstract
Introduction: Baricitinib is an oral selective inhibitor of Janus kinase (JAK) 1 and JAK 2, which has demonstrated significant efficacy in patients with moderately to severely active rheumatoid arthritis (RA). This analysis aims to describe the efficacy and safety of baricitinib in Chinese RA patients with an inadequate response to methotrexate (MTX-IR), and to analyze the effects of baseline characteristics on the efficacy of baricitinib treatment.
Methods: In this 52-week, randomized, double-blind, placebo-controlled study, 231 Chinese patients with moderately to severely active RA who had MTX-IR were randomly assigned to placebo (n = 115) or baricitinib 4 mg once daily (n = 116). The primary endpoint was American College of Rheumatology 20% (ACR20) response at week 12. Other efficacy measures included ACR50, ACR70, Physician's Global Assessment of Disease Activity, Patient's Global Assessment of Disease Activity, patient's assessment of pain, Disease Activity Score in 28 joints using high-sensitivity C-reactive protein, remission and low disease activity rates according to Simplified Disease Activity Index or Clinical Disease Activity Index, Health Assessment Questionnaire-Disability Index, and mean duration and severity of morning joint stiffness, worst tiredness and worst joint pain were analyzed. Additionally, subgroup analyses were performed across baseline characteristics.
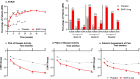
Results: Statistically significant improvement in ACR20 response was achieved with baricitinib at week 12 (53.4 vs. 22.6%, p = 0.001) in Chinese patients, compared to placebo. Most of the secondary objectives were met with statistically significant improvements. Efficacy of baricitinib was irrespective of patient demographics and baseline characteristics. Safety events were similar between the baricitinib and placebo groups.
Conclusions: The efficacy of baricitinib 4 mg in Chinese patients with moderately to severely active RA and prior MTX-IR was clinically significant compared to placebo regardless of baseline characteristics. Baricitinib was well tolerated with an acceptable safety profile during the full study period.
Trial registration: NCT02265705.
Keywords: Arthritis; Baricitinib; China; Rheumatoid.
Figures


References
-
- Chinese Rheumatology Association 2018 Chinese guideline for the diagnosis and treatment of rheumatoid arthritis. Zhonghua Nei Ke Za Zhi. 2018;57:242–251. - PubMed
-
- Yu C, Li M, Duan X, et al. Chinese registry of rheumatoid arthritis (CREDIT): I. Introduction and prevalence of remission in Chinese patients with rheumatoid arthritis. Clin Exp Rheumatol. 2018;36:836–840. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

