Maternal low-protein diet on the last week of pregnancy contributes to insulin resistance and β-cell dysfunction in the mouse offspring
- PMID: 32877242
- PMCID: PMC7717124
- DOI: 10.1152/ajpregu.00284.2019
Maternal low-protein diet on the last week of pregnancy contributes to insulin resistance and β-cell dysfunction in the mouse offspring
Abstract
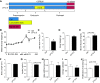
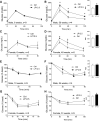
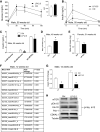
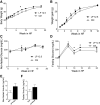
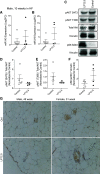
Maternal low-protein diet (LP) throughout gestation affects pancreatic β-cell fraction of the offspring at birth, thus increasing their susceptibility to metabolic dysfunction and type 2 diabetes in adulthood. The present study sought to strictly examine the effects of LP during the last week of gestation (LP12.5) alone as a developmental window for β-cell programming and metabolic dysfunction in adulthood. Islet morphology analysis revealed normal β-cell fraction in LP12.5 newborns. Normal glucose tolerance was observed in 6- to 8-wk-old male and female LP12.5 offspring. However, male LP12.5 offspring displayed glucose intolerance and reduced insulin sensitivity associated with β-cell dysfunction with aging. High-fat diet exposure of metabolically normal 12-wk-old male LP12.5 induced glucose intolerance due to increased body weight, insulin resistance, and insufficient β-cell mass adaptation despite higher insulin secretion. Assessment of epigenetic mechanisms through microRNAs (miRs) by a real-time PCR-based microarray in islets revealed elevation in miRs that regulate insulin secretion (miRs 342, 143), insulin resistance (miR143), and obesity (miR219). In the islets, overexpression of miR143 reduced insulin secretion in response to glucose. In contrast to the model of LP exposure throughout pregnancy, islet protein levels of mTOR and pancreatic and duodenal homeobox 1 were normal in LP12.5 islets. Collectively, these data suggest that LP diet during the last week of pregnancy is critical and sufficient to induce specific and distinct developmental programming effects of tissues that control glucose homeostasis, thus causing permanent changes in specific set of microRNAs that may contribute to the overall vulnerability of the offspring to obesity, insulin resistance, and type 2 diabetes.
Keywords: diabetes; fetal nutrition disorders; fetal programming; islet biology; low-protein diet; β-cell function.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- DK073716/GF/NIH HHS/United States
- R21HD100840/GF/NIH HHS/United States
- R21 DK112144/DK/NIDDK NIH HHS/United States
- P30 DK089503/DK/NIDDK NIH HHS/United States
- I01 BX002728/BX/BLRD VA/United States
- R01DK084236/GF/NIH HHS/United States
- N02 DK062876/DK/NIDDK NIH HHS/United States
- R21 HD100840/HD/NICHD NIH HHS/United States
- K08 DK102526/DK/NIDDK NIH HHS/United States
- R01DK115720/GF/NIH HHS/United States
- R21DK112144/GF/NIH HHS/United States
- RO1DK62876/GF/NIH HHS/United States
- R01 DK084236/DK/NIDDK NIH HHS/United States
- R01 DK073716/DK/NIDDK NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- R01 DK115720/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

