Role of e-cigarettes and pharmacotherapy during attempts to quit cigarette smoking: The PATH Study 2013-16
- PMID: 32877429
- PMCID: PMC7467279
- DOI: 10.1371/journal.pone.0237938
Role of e-cigarettes and pharmacotherapy during attempts to quit cigarette smoking: The PATH Study 2013-16
Abstract
Background: More smokers report using e-cigarettes to help them quit than FDA-approved pharmacotherapy.
Objective: To assess the association of e-cigarettes with future abstinence from cigarette and tobacco use.
Design: Cohort study of US sample, with annual follow-up.
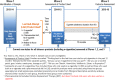
Participants: US adult (ages 18+) daily cigarette smokers identified at Wave 1 (W1; 2013-14) of the PATH Study, who reported a quit attempt before W2 and completed W3 (n = 2443).
Exposures: Use of e-cigarettes, pharmacotherapy (including nicotine replacement therapy), or no product for last quit attempt (LQA), and current daily e-cigarette use at W2.
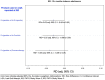
Analysis: Propensity score matching (PSM) of groups using different methods to quit.
Outcome measures: 12+ months abstinence at W3 from cigarettes and from all tobacco (including e-cigarettes). 30+ days abstinence at W3 was a secondary outcome.
Results: Among daily smokers with an LQA, 23.5% used e-cigarettes, 19.3% used pharmacotherapy only (including NRT) and 57.2% used no product. Cigarette abstinence for 12+ months at W3 was ~10% in each group. Half of the cigarette abstainers in the e-cigarette group were using e-cigarettes at W3. Different methods to help quitting had statistically comparable 12+ month cigarette abstinence at W3 (e-cigarettes vs no product: Risk Difference (RD) = 0.01, 95% CI: -0.04 to 0.06; e-cigarettes vs pharmacotherapy: RD = 0.02, 95% CI:-0.04 to 0.09). Likewise, daily e-cigarette users at W2 did not show a cessation benefit over comparable no-e-cigarette users and this finding was robust to sensitivity analyses. Abstinence for 30+ days at W3 was also similar across products.
Limitations: The frequency of e-cigarette use during the LQA was not assessed, nor was it possible to assess continuous abstinence from the LQA.
Conclusion: Among US daily smokers who quit cigarettes in 2014-15, use of e-cigarettes in that attempt compared to approved cessation aids or no products showed similar abstinence rates 1-2 years later.
Conflict of interest statement
KMC has received payment as a consultant to Pfizer, Inc., for service on an external advisory panel to assess ways to improve smoking cessation delivery in health care settings. KMC also has served as paid expert witness in litigation filed against the tobacco industry. MG receives fees for serving on an advisory board from Johnson & Johnson and grant support from Pfizer. WC reports long-term stock holdings in General Electric Company, 3M Company, and Pfizer Incorporated, unrelated to this manuscript. Westat is a commercial, employee-owned research corporation. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- US Department of Health and Human Services. Reducing Tobacco Use: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2000.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

