A radioligand binding assay for the insulin-like growth factor 2 receptor
- PMID: 32877466
- PMCID: PMC7467306
- DOI: 10.1371/journal.pone.0238393
A radioligand binding assay for the insulin-like growth factor 2 receptor
Abstract
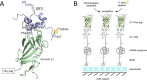
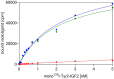
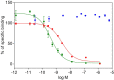
Insulin-like growth factors 2 and 1 (IGF2 and IGF1) and insulin are closely related hormones that are responsible for the regulation of metabolic homeostasis, development and growth of the organism. Physiological functions of insulin and IGF1 are relatively well-studied, but information about the role of IGF2 in the body is still sparse. Recent discoveries called attention to emerging functions of IGF2 in the brain, where it could be involved in processes of learning and memory consolidation. It was also proposed that these functions could be mediated by the receptor for IGF2 (IGF2R). Nevertheless, little is known about the mechanism of signal transduction through this receptor. Here we produced His-tagged domain 11 (D11), an IGF2-binding element of IGF2R; we immobilized it on the solid support through a well-defined sandwich, consisting of neutravidin, biotin and synthetic anti-His-tag antibodies. Next, we prepared specifically radiolabeled [125I]-monoiodotyrosyl-Tyr2-IGF2 and optimized a sensitive and robust competitive radioligand binding assay for determination of the nanomolar binding affinities of hormones for D11 of IGF2. The assay will be helpful for the characterization of new IGF2 mutants to study the functions of IGF2R and the development of new compounds for the treatment of neurological disorders.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Effect of kainic acid treatment on insulin-like growth factor-2 receptors in the IGF2-deficient adult mouse brain.Brain Res. 2007 Feb 2;1131(1):77-87. doi: 10.1016/j.brainres.2006.11.022. Epub 2006 Dec 20. Brain Res. 2007. PMID: 17184742
-
Insulin-like growth factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine granulosa cells through the IGF1 receptor: role of follicle-stimulating hormone and IGF2 receptor.Biol Reprod. 2007 Jul;77(1):18-27. doi: 10.1095/biolreprod.106.058230. Epub 2007 Mar 14. Biol Reprod. 2007. PMID: 17360960
-
IGF2 actions on trophoblast in human placenta are regulated by the insulin-like growth factor 2 receptor, which can function as both a signaling and clearance receptor.Biol Reprod. 2011 Mar;84(3):440-6. doi: 10.1095/biolreprod.110.088195. Epub 2010 Oct 27. Biol Reprod. 2011. PMID: 20980691 Free PMC article.
-
Role of IGF-binding proteins in regulating IGF responses to changes in metabolism.J Mol Endocrinol. 2018 Jul;61(1):T139-T169. doi: 10.1530/JME-18-0016. Epub 2018 Mar 21. J Mol Endocrinol. 2018. PMID: 29563157 Review.
-
Insulin-like growth factor 2 in development and disease: a mini-review.Gerontology. 2013;59(3):240-9. doi: 10.1159/000343995. Epub 2012 Dec 20. Gerontology. 2013. PMID: 23257688 Review.
Cited by
-
Late-stage labeling of diverse peptides and proteins with iodine-125.J Pharm Anal. 2025 Jul;15(7):101198. doi: 10.1016/j.jpha.2025.101198. Epub 2025 Jan 17. J Pharm Anal. 2025. PMID: 40741338 Free PMC article. Review.
-
The final walk with preptin.PLoS One. 2024 Sep 12;19(9):e0309726. doi: 10.1371/journal.pone.0309726. eCollection 2024. PLoS One. 2024. PMID: 39264940 Free PMC article.
-
Non-glycosylated IGF2 prohormones are more mitogenic than native IGF2.Commun Biol. 2023 Aug 19;6(1):863. doi: 10.1038/s42003-023-05239-6. Commun Biol. 2023. PMID: 37598269 Free PMC article.
-
Pan-cancer analysis of oncogenic role of insulin-like growth factor-binding proteins and validation in ovarian cancer.Cancer Med. 2023 Jul;12(13):14833-14850. doi: 10.1002/cam4.6073. Epub 2023 May 18. Cancer Med. 2023. PMID: 37199034 Free PMC article.
-
Design of Potent Mannose-6-Phosphate Derivatives as Ligands for CI-M6P/IGF2R Using Fluorescence Polarization Assay.Chemistry. 2025 Jul 22;31(41):e202500973. doi: 10.1002/chem.202500973. Epub 2025 May 20. Chemistry. 2025. PMID: 40356531 Free PMC article.
References
-
- Efstratiadis A. Genetics of mouse growth. Int J Dev Biol. 1998;42(7):955–76. PMID: ISI:000076795600017. - PubMed
-
- Eggenschwiler J, Ludwig T, Fisher P, Leighton PA, Tilghman SM, Efstratiadis A. Mouse mutant embryos overexpressing IGF-II exhibit phenotypic features of the Beckwith-Wiedemann and Simpson-Golabi-Behmel syndromes. Genes & Development. 1997;11(23):3128–42. 10.1101/gad.11.23.3128 PMID: ISI:A1997YL52600004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

