Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition
- PMID: 32877502
- PMCID: PMC7596849
- DOI: 10.1182/blood.2020008248
Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition
Abstract
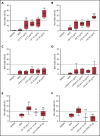
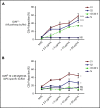
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly contagious respiratory virus that can lead to venous/arterial thrombosis, stroke, renal failure, myocardial infarction, thrombocytopenia, and other end-organ damage. Animal models demonstrating end-organ protection in C3-deficient mice and evidence of complement activation in humans have led to the hypothesis that SARS-CoV-2 triggers complement-mediated endothelial damage, but the mechanism is unclear. Here, we demonstrate that the SARS-CoV-2 spike protein (subunit 1 and 2), but not the N protein, directly activates the alternative pathway of complement (APC). Complement-dependent killing using the modified Ham test is blocked by either C5 or factor D inhibition. C3 fragments and C5b-9 are deposited on TF1PIGAnull target cells, and complement factor Bb is increased in the supernatant from spike protein-treated cells. C5 inhibition prevents the accumulation of C5b-9 on cells, but not C3c; however, factor D inhibition prevents both C3c and C5b-9 accumulation. Addition of factor H mitigates the complement attack. In conclusion, SARS-CoV-2 spike proteins convert nonactivator surfaces to activator surfaces by preventing the inactivation of the cell-surface APC convertase. APC activation may explain many of the clinical manifestations (microangiopathy, thrombocytopenia, renal injury, and thrombophilia) of COVID-19 that are also observed in other complement-driven diseases such as atypical hemolytic uremic syndrome and catastrophic antiphospholipid antibody syndrome. C5 inhibition prevents accumulation of C5b-9 in vitro but does not prevent upstream complement activation in response to SARS-CoV-2 spike proteins.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: R.A.B. has served on advisory board for Alexion Pharmaceutical Inc. The remaining authors declare no competing financial interests.
Figures





Comment in
-
Targeting complement activation in COVID-19.Blood. 2020 Oct 29;136(18):2000-2001. doi: 10.1182/blood.2020008925. Blood. 2020. PMID: 33119763 Free PMC article.
-
Thrombotic events after AstraZeneca vaccine: What if it was related to dysfunctional immune response?Therapie. 2021 Jul-Aug;76(4):367-369. doi: 10.1016/j.therap.2021.04.003. Epub 2021 Apr 20. Therapie. 2021. PMID: 33892936 Free PMC article. No abstract available.
References
-
- Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005;24(suppl 11):S223-S227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

