Phase 1/2a study of 177Lu-lilotomab satetraxetan in relapsed/refractory indolent non-Hodgkin lymphoma
- PMID: 32877524
- PMCID: PMC7479948
- DOI: 10.1182/bloodadvances.2020002583
Phase 1/2a study of 177Lu-lilotomab satetraxetan in relapsed/refractory indolent non-Hodgkin lymphoma
Abstract
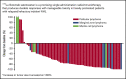
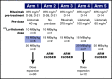
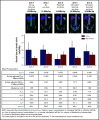
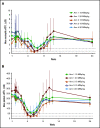
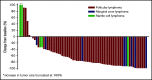
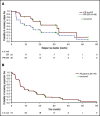
For patients with indolent non-Hodgkin lymphoma who fail initial anti-CD20-based immunochemotherapy or develop relapsed or refractory disease, there remains a significant unmet clinical need for new therapeutic approaches to improve outcomes and quality of life. 177Lu-lilotomab satetraxetan is a next-generation single-dose CD37-directed radioimmunotherapy (RIT) which was investigated in a phase 1/2a study in 74 patients with relapsed/refractory indolent non-Hodgkin B-cell lymphoma, including 57 patients with follicular lymphoma (FL). To improve targeting of 177Lu-lilotomab satetraxetan to tumor tissue and decrease hematologic toxicity, its administration was preceded by the anti-CD20 monoclonal antibody rituximab and the "cold" anti-CD37 antibody lilotomab. The most common adverse events (AEs) were reversible grade 3/4 neutropenia (31.6%) and thrombocytopenia (26.3%) with neutrophil and platelet count nadirs 5 to 7 weeks after RIT. The most frequent nonhematologic AE was grade 1/2 nausea (15.8%). With a single administration, the overall response rate was 61% (65% in patients with FL), including 30% complete responses. For FL with ≥2 prior therapies (n = 37), the overall response rate was 70%, including 32% complete responses. For patients with rituximab-refractory FL ≥2 prior therapies (n = 21), the overall response rate was 67%, and the complete response rate was 24%. The overall median duration of response was 13.6 months (32.0 months for patients with a complete response). 177Lu-lilotomab satetraxetan may provide a valuable alternative treatment approach in relapsed/refractory non-Hodgkin lymphoma, particularly in patients with comorbidities unsuitable for more intensive approaches. This trial was registered at www.clinicaltrials.gov as #NCT01796171.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.K. declares advisory board participation for Nordic Nanovector ASA. T.I. declares advisory board participation for Nordic Nanovector ASA, Roche, and Takeda. S.S. declares lecture honoraria from Novartis and Sanofi Genzyme. W.J. declares research funding/grants from Nordic Nanovector ASA. U.-M.F. declares advisory board participation for Takeda and Roche. H.H. declares advisory board participation for Nordic Nanovector ASA, Novartis, Roche, Gilead, and Takeda and lecture honoraria from Novartis. J.D. and V.P. are employees of Nordic Nanovector ASA and owners of performance share units. J.D. also declares ownership of shares. L.R. was an employee of Nordic Nanovector ASA at the time of this study. The remaining authors declare no competing financial interests.
Figures
References
-
- McLaughlin P, Grillo-López AJ, Link BK, et al. . Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825-2833. - PubMed
-
- Sehn LH, Chua N, Mayer J, et al. . Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17(8):1081-1093. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

