Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19
- PMID: 32877699
- PMCID: PMC7437499
- DOI: 10.1016/j.cell.2020.08.025
Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19
Abstract
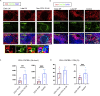
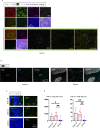
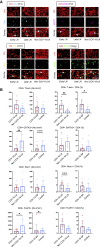
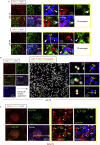
Humoral responses in coronavirus disease 2019 (COVID-19) are often of limited durability, as seen with other human coronavirus epidemics. To address the underlying etiology, we examined post mortem thoracic lymph nodes and spleens in acute SARS-CoV-2 infection and observed the absence of germinal centers and a striking reduction in Bcl-6+ germinal center B cells but preservation of AID+ B cells. Absence of germinal centers correlated with an early specific block in Bcl-6+ TFH cell differentiation together with an increase in T-bet+ TH1 cells and aberrant extra-follicular TNF-α accumulation. Parallel peripheral blood studies revealed loss of transitional and follicular B cells in severe disease and accumulation of SARS-CoV-2-specific "disease-related" B cell populations. These data identify defective Bcl-6+ TFH cell generation and dysregulated humoral immune induction early in COVID-19 disease, providing a mechanistic explanation for the limited durability of antibody responses in coronavirus infections, and suggest that achieving herd immunity through natural infection may be difficult.
Keywords: COVID-19; SARS-CoV-2; T follicular helper cells; TNF-α; cytokine dysregulation; double-negative B cells; extra-follicular B cells; germinal centers; humoral immunity; plasmablasts.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests S.P. is on the SAB of Abpro Inc. and Pulsar Biopharma. G.A. is founder of Seromyx Systems Inc.
Figures
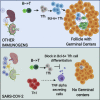
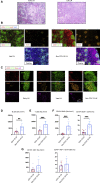
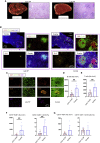

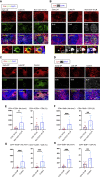






Update of
-
The Loss of Bcl-6 Expressing T Follicular Helper Cells and the Absence of Germinal Centers in COVID-19.SSRN [Preprint]. 2020 Jul 16:3652322. doi: 10.2139/ssrn.3652322. SSRN. 2020. Update in: Cell. 2020 Oct 1;183(1):143-157.e13. doi: 10.1016/j.cell.2020.08.025. PMID: 32742244 Free PMC article. Updated. Preprint.
Comment in
-
COVID-19 Makes B Cells Forget, but T Cells Remember.Cell. 2020 Oct 1;183(1):13-15. doi: 10.1016/j.cell.2020.09.013. Epub 2020 Sep 4. Cell. 2020. PMID: 32976799 Free PMC article.
References
-
- Buja L.M., Wolf D.A., Zhao B., Akkanti B., McDonald M., Lelenwa L., Reilly N., Ottaviani G., Elghetany M.T., Trujillo D.O. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc. Pathol. 2020;48:107233. - PMC - PubMed
-
- Ecker R.C., Steiner G.E. Microscopy-based multicolor tissue cytometry at the single-cell level. Cytometry A. 2004;59:182–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI153098/AI/NIAID NIH HHS/United States
- U19 AI110495/AI/NIAID NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- T32 AI007245/AI/NIAID NIH HHS/United States
- U19 AI057229/AI/NIAID NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- R01 AI146779/AI/NIAID NIH HHS/United States
- T32 AI007529/AI/NIAID NIH HHS/United States
- T32 GM008313/GM/NIGMS NIH HHS/United States
- R01 AI124378/AI/NIAID NIH HHS/United States
- R01 AI137057/AI/NIAID NIH HHS/United States
- F30 NS065566/NS/NINDS NIH HHS/United States
- DP2 DA042422/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

