Interferon Inducing Porcine Reproductive and Respiratory Syndrome Virus Vaccine Candidate Protected Piglets from HP-PRRSV Challenge and Evoke a Higher Level of Neutralizing Antibodies Response
- PMID: 32877992
- PMCID: PMC7565719
- DOI: 10.3390/vaccines8030490
Interferon Inducing Porcine Reproductive and Respiratory Syndrome Virus Vaccine Candidate Protected Piglets from HP-PRRSV Challenge and Evoke a Higher Level of Neutralizing Antibodies Response
Abstract
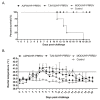
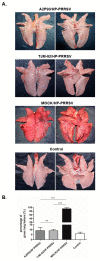
Although widespread administration of attenuated porcine reproductive and respiratory syndrome virus (PRRSV) vaccines has been implemented since they first became commercially available two decades ago, PRRSV infection prevalence in swine herds remains high. The limited success of PRRSV vaccines is partly due to the well-established fact that a given vaccine strain confers only partial or no protection against heterologous strains. In our past work, A2MC2-P90, a novel PRRSV vaccine candidate that induced a type I IFNs response in vitro, conferred complete protection against challenge with genetically heterologous PRRSV strains. Here we assessed the ability of the PRRSV vaccine candidate A2MC2-P90 to protect piglets against the HP-PRRSV challenge and compared its efficacy to that of a licensed HP-PRRSV-specific vaccine (TJM-F92) assessed in parallel. A2MC2-P90 provided vaccinated piglets with 100% protection from a lethal challenge with extremely virulent HP-PRRSV-XJA1, while 100% mortality was observed for unvaccinated piglets by day 21 post-challenge. Notably, comparison of partial sequence (GP5) of XJA1 to A2MC2-P90 suggested there was only 88.7% homology. When comparing post-HP-PRRSV challenge responses between piglets administered A2AMC2-P90 versus those immunized with licensed vaccine TJM-F92, A2MC2-P90-vaccinated piglets rapidly developed a stronger protective humoral immune response, as evidenced by much higher titers of neutralizing antibodies, more rapid clearance of viremia and less nasal virus shedding. In conclusion, our data suggest that this novel vaccine candidate A2MC2-P90 has improved protection spectrum against heterologous HP-PRRSV strains.
Keywords: HP-PRRSV; IFN induction; PRRSV; modified live vaccines; neutralizing antibodies; protection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
An interferon inducing porcine reproductive and respiratory syndrome virus vaccine candidate elicits protection against challenge with the heterologous virulent type 2 strain VR-2385 in pigs.Vaccine. 2017 Jan 3;35(1):125-131. doi: 10.1016/j.vaccine.2016.11.020. Epub 2016 Nov 18. Vaccine. 2017. PMID: 27876202
-
A potent CD8 T-cell response may be associated with partial cross-protection conferred by an attenuated Chinese HP-PRRSV vaccine against NADC30-like PRRSV challenge.J Gen Virol. 2023 May;104(5). doi: 10.1099/jgv.0.001850. J Gen Virol. 2023. PMID: 37159409
-
Efficacy evaluation of three modified-live PRRS vaccines against a local strain of highly pathogenic porcine reproductive and respiratory syndrome virus.Vet Microbiol. 2019 Feb;229:117-123. doi: 10.1016/j.vetmic.2018.12.016. Epub 2018 Dec 17. Vet Microbiol. 2019. PMID: 30642586
-
Innate and adaptive immunity against Porcine Reproductive and Respiratory Syndrome Virus.Vet Immunol Immunopathol. 2015 Sep 15;167(1-2):1-14. doi: 10.1016/j.vetimm.2015.07.003. Epub 2015 Jul 17. Vet Immunol Immunopathol. 2015. PMID: 26209116 Free PMC article. Review.
-
Testable Candidate Immune Correlates of Protection for Porcine Reproductive and Respiratory Syndrome Virus Vaccination.Vaccines (Basel). 2023 Mar 5;11(3):594. doi: 10.3390/vaccines11030594. Vaccines (Basel). 2023. PMID: 36992179 Free PMC article. Review.
Cited by
-
Evaluation of porcine GM-CSF during PRRSV infection in vitro and in vivo indicating a protective role of GM-CSF related with M1 biased activation in alveolar macrophage during PRRSV infection.Front Immunol. 2022 Oct 19;13:967338. doi: 10.3389/fimmu.2022.967338. eCollection 2022. Front Immunol. 2022. PMID: 36341451 Free PMC article.
-
Research Progress on the Development of Porcine Reproductive and Respiratory Syndrome Vaccines.Vet Sci. 2023 Jul 28;10(8):491. doi: 10.3390/vetsci10080491. Vet Sci. 2023. PMID: 37624278 Free PMC article. Review.
-
A porcine reproductive and respiratory syndrome virus (PRRSV)-specific IgM as a novel adjuvant for an inactivated PRRSV vaccine improves protection efficiency and enhances cell-mediated immunity against heterologous PRRSV challenge.Vet Res. 2022 Aug 19;53(1):65. doi: 10.1186/s13567-022-01082-5. Vet Res. 2022. PMID: 35986391 Free PMC article.
-
Systems Immunology Analyses Following Porcine Respiratory and Reproductive Syndrome Virus Infection and Vaccination.Front Immunol. 2021 Dec 16;12:779747. doi: 10.3389/fimmu.2021.779747. eCollection 2021. Front Immunol. 2021. PMID: 34975868 Free PMC article.
References
-
- Adams M.J., Lefkowitz E.J., King A.M., Harrach B., Harrison R.L., Knowles N.J., Kropinski A.M., Krupovic M., Kuhn J.H., Mushegian A.R., et al. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2016) Arch. Virol. 2016;161:2921–2949. doi: 10.1007/s00705-016-2977-6. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

