Breast and Gut Microbiota Action Mechanisms in Breast Cancer Pathogenesis and Treatment
- PMID: 32878124
- PMCID: PMC7565530
- DOI: 10.3390/cancers12092465
Breast and Gut Microbiota Action Mechanisms in Breast Cancer Pathogenesis and Treatment
Abstract
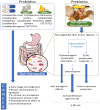
In breast cancer (BC) the employment of sequencing technologies for metagenomic analyses has allowed not only the description of the overall metagenomic landscape but also the specific microbial changes and their functional implications. Most of the available data suggest that BC is related to bacterial dysbiosis in both the gut microenvironment and breast tissue. It is hypothesized that changes in the composition and functions of several breast and gut bacterial taxa may contribute to BC development and progression through several pathways. One of the most prominent roles of gut microbiota is the regulation of steroid-hormone metabolism, such as estrogens, a component playing an important role as risk factor in BC development, especially in postmenopausal women. On the other hand, breast and gut resident microbiota are the link in the reciprocal interactions between cancer cells and their local environment, since microbiota are capable of modulating mucosal and systemic immune responses. Several in vivo and in vitro studies show remarkable evidence that diet, probiotics and prebiotics could exert important anticarcinogenic effects in BC. Moreover, gut microbiota have an important role in the metabolism of chemotherapeutic drugs and in the activity of immunogenic chemotherapies since they are a potential dominant mediator in the response to cancer therapy. Then, the microbiome impact in BC is multi-factorial, and the gut and breast tissue bacteria population could be important in regulating the local immune system, in tumor formation and progression and in therapy response and/or resistance.
Keywords: anticancer therapy; breast cancer; epigenetic modulation; estrobolome; immune responds; inflammation; microbiota; prebiotics; probiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sorlie T., Tibshirani R., Parker J., Hastie T., Marron J.S., Nobel A., Deng S., Johnsen H., Pesich R., Geisler S., et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. - DOI - PMC - PubMed

