Antibody and Local Cytokine Response to Respiratory Syncytial Virus Infection in Community-Dwelling Older Adults
- PMID: 32878928
- PMCID: PMC7471002
- DOI: 10.1128/mSphere.00577-20
Antibody and Local Cytokine Response to Respiratory Syncytial Virus Infection in Community-Dwelling Older Adults
Abstract
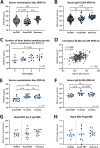
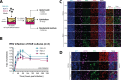
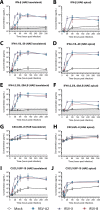
Respiratory syncytial virus (RSV) is increasingly recognized for causing severe morbidity and mortality in older adults, but there are few studies on the RSV-induced immune response in this population. Information on the immunological processes at play during RSV infection in specific risk groups is essential for the rational and targeted design of novel vaccines and therapeutics. Here, we assessed the antibody and local cytokine response to RSV infection in community-dwelling older adults (≥60 years of age). During three winters, serum and nasopharyngeal swab samples were collected from study participants during acute respiratory infection and recovery. RSV IgG enzyme-linked immunosorbent assays (ELISA) and virus neutralization assays were performed on serum samples from RSV-infected individuals (n = 41) and controls (n = 563 and n = 197, respectively). Nasal RSV IgA and cytokine concentrations were determined using multiplex immunoassays in a subset of participants. An in vitro model of differentiated primary bronchial epithelial cells was used to assess RSV-induced cytokine responses over time. A statistically significant increase in serum neutralization titers and IgG concentrations was observed in RSV-infected participants compared to controls. During acute RSV infection, a statistically significant local upregulation of beta interferon (IFN-β), IFN-λ1, IFN-γ, interleukin 1β (IL-1β), tumor necrosis factor alpha (TNF-α), IL-6, IL-10, CXCL8, and CXCL10 was found. IFN-β, IFN-λ1, CXCL8, and CXCL10 were also upregulated in the epithelial model upon RSV infection. In conclusion, this study provides novel insights into the basic immune response to RSV infection in an important and understudied risk population, providing leads for future studies that are essential for the prevention and treatment of severe RSV disease in older adults.IMPORTANCE Respiratory syncytial virus (RSV) can cause severe morbidity and mortality in certain risk groups, especially infants and older adults. Currently no (prophylactic) treatment is available, except for a partially effective yet highly expensive monoclonal antibody. RSV therefore remains a major public health concern. To allow targeted development of novel vaccines and therapeutics, it is of great importance to understand the immunological mechanisms that underlie (protection from) severe disease in specific risk populations. Since most RSV-related studies focus on infants, there are only very limited data available concerning the response to RSV in the elderly population. Therefore, in this study, RSV-induced antibody responses and local cytokine secretion were assessed in community-dwelling older adults. These data provide novel insights that will benefit ongoing efforts to design safe and effective prevention and treatment strategies for RSV in an understudied risk group.
Keywords: IgA; IgG; cytokine; elderly; interferon; mucosa.
Copyright © 2020 Yu et al.
Figures



References
-
- Shi T, Arnott A, Semogas I, Falsey AR, Openshaw P, Wedzicha JA, Campbell H, Nair H. 2019. The etiological role of common respiratory viruses in acute respiratory infections in older adults: a systematic review and meta-analysis. J Infect Dis doi:10.1093/infdis/jiy662. Epub ahead of print. - DOI - PMC - PubMed
-
- Shi T, Denouel A, Tietjen AK, Campbell I, Moran E, Li X, Campbell H, Demont C, Nyawanda BO, Chu HY, Stoszek SK, Krishnan A, Openshaw P, Falsey AR, Nair H, RescEU Investigators. 2019. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis doi:10.1093/infdis/jiz059. Epub ahead of print. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

