Amalgam regulates the receptor tyrosine kinase pathway through Sprouty in glial cell development in the Drosophila larval brain
- PMID: 32878945
- PMCID: PMC7541346
- DOI: 10.1242/jcs.250837
Amalgam regulates the receptor tyrosine kinase pathway through Sprouty in glial cell development in the Drosophila larval brain
Abstract
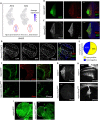
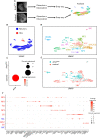
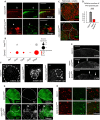
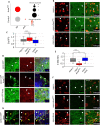
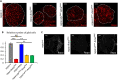
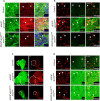
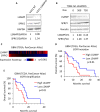
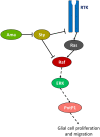
The receptor tyrosine kinase (RTK) pathway plays an essential role in development and disease by controlling cell proliferation and differentiation. Here, we profile the Drosophila larval brain by single-cell RNA-sequencing and identify Amalgam (Ama), which encodes a cell adhesion protein of the immunoglobulin IgLON family, as regulating the RTK pathway activity during glial cell development. Depletion of Ama reduces cell proliferation, affects glial cell type composition and disrupts the blood-brain barrier (BBB), which leads to hemocyte infiltration and neuronal death. We show that Ama depletion lowers RTK activity by upregulating Sprouty (Sty), a negative regulator of the RTK pathway. Knockdown of Ama blocks oncogenic RTK signaling activation in the Drosophila glioma model and halts malignant transformation. Finally, knockdown of a human ortholog of Ama, LSAMP, results in upregulation of SPROUTY2 in glioblastoma cell lines, suggesting that the relationship between Ama and Sty is conserved.
Keywords: Blood–brain barrier; Drosophila; LSAMP; Receptor tyrosine kinase; Single-cell RNA-sequencing; Sprouty.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

