Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors
- PMID: 32878978
- PMCID: PMC9126033
- DOI: 10.1126/scitranslmed.aaz1863
Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors
Abstract
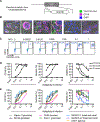
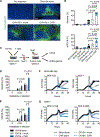
Chimeric antigen receptor (CAR)-engineered T cell therapy for solid tumors is limited by the lack of both tumor-restricted and homogeneously expressed tumor antigens. Therefore, we engineered an oncolytic virus to express a nonsignaling, truncated CD19 (CD19t) protein for tumor-selective delivery, enabling targeting by CD19-CAR T cells. Infecting tumor cells with an oncolytic vaccinia virus coding for CD19t (OV19t) produced de novo CD19 at the cell surface before virus-mediated tumor lysis. Cocultured CD19-CAR T cells secreted cytokines and exhibited potent cytolytic activity against infected tumors. Using several mouse tumor models, delivery of OV19t promoted tumor control after CD19-CAR T cell administration. OV19t induced local immunity characterized by tumor infiltration of endogenous and adoptively transferred T cells. CAR T cell-mediated tumor killing also induced release of virus from dying tumor cells, which propagated tumor expression of CD19t. Our study features a combination immunotherapy approach using oncolytic viruses to promote de novo CAR T cell targeting of solid tumors.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures




Comment in
-
Combination Therapy for Solid Tumors: Taking a Classic CAR on New Adventures.Cancer Cell. 2020 Nov 9;38(5):621-623. doi: 10.1016/j.ccell.2020.10.003. Epub 2020 Oct 15. Cancer Cell. 2020. PMID: 33064993
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

