C9orf72 poly(GR) aggregation induces TDP-43 proteinopathy
- PMID: 32878979
- PMCID: PMC7989020
- DOI: 10.1126/scitranslmed.abb3774
C9orf72 poly(GR) aggregation induces TDP-43 proteinopathy
Abstract
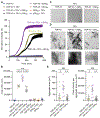
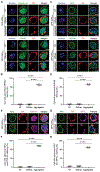
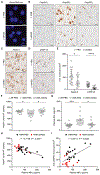
TAR DNA-binding protein 43 (TDP-43) inclusions are a pathological hallmark of frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS), including cases caused by G4C2 repeat expansions in the C9orf72 gene (c9FTD/ALS). Providing mechanistic insight into the link between C9orf72 mutations and TDP-43 pathology, we demonstrated that a glycine-arginine repeat protein [poly(GR)] translated from expanded G4C2 repeats was sufficient to promote aggregation of endogenous TDP-43. In particular, toxic poly(GR) proteins mediated sequestration of full-length TDP-43 in an RNA-independent manner to induce cytoplasmic TDP-43 inclusion formation. Moreover, in GFP-(GR)200 mice, poly(GR) caused the mislocalization of nucleocytoplasmic transport factors and nuclear pore complex proteins. These mislocalization events resulted in the aberrant accumulation of endogenous TDP-43 in the cytoplasm where it co-aggregated with poly(GR). Last, we demonstrated that treating G4C2 repeat-expressing mice with repeat-targeting antisense oligonucleotides lowered poly(GR) burden, which was accompanied by reduced TDP-43 pathology and neurodegeneration, including lowering of plasma neurofilament light (NFL) concentration. These results contribute to clarification of the mechanism by which poly(GR) drives TDP-43 proteinopathy, confirm that G4C2-targeted therapeutics reduce TDP-43 pathology in vivo, and demonstrate that alterations in plasma NFL provide insight into the therapeutic efficacy of disease-modifying treatments.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


References
-
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM-Y, Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006). - PubMed
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T, TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun 351, 602–611 (2006). - PubMed
-
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung G-YR, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R, Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256 (2011). - PMC - PubMed
-
- Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita V-M, Kaivorinne A-L, Hölttä-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chiò A, Restagno G, Borghero G, Sabatelli M; The ITALSGEN Consortium, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ, A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268 (2011). - PMC - PubMed
-
- Lagier-Tourenne C, Baughn M, Rigo F, Sun S, Liu P, Li H-R, Jiang J, Watt AT, Chun S, Katz M, Qiu J, Sun Y, Ling S-C, Zhu Q, Polymenidou M, Drenner K, Artates JW, McAlonis-Downes M, Markmiller S, Hutt KR, Pizzo DP, Cady J, Harms MB, Baloh RH, Vandenberg SR, Yeo GW, Fu X-D, Bennett CF, Cleveland DW, Ravits J, Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc. Natl. Acad. Sci. U.S.A 110, E4530–E4539 (2013). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

