FGF/FGFR signaling in health and disease
- PMID: 32879300
- PMCID: PMC7468161
- DOI: 10.1038/s41392-020-00222-7
FGF/FGFR signaling in health and disease
Abstract
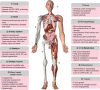
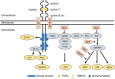
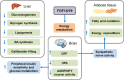
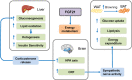
Growing evidences suggest that the fibroblast growth factor/FGF receptor (FGF/FGFR) signaling has crucial roles in a multitude of processes during embryonic development and adult homeostasis by regulating cellular lineage commitment, differentiation, proliferation, and apoptosis of various types of cells. In this review, we provide a comprehensive overview of the current understanding of FGF signaling and its roles in organ development, injury repair, and the pathophysiology of spectrum of diseases, which is a consequence of FGF signaling dysregulation, including cancers and chronic kidney disease (CKD). In this context, the agonists and antagonists for FGF-FGFRs might have therapeutic benefits in multiple systems.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Wiedemann M, Trueb B. Characterization of a novel protein (FGFRL1) from human cartilage related to FGF receptors. Genomics. 2000;69:275–279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

