Metabolic alterations in Parkinson's disease astrocytes
- PMID: 32879386
- PMCID: PMC7468111
- DOI: 10.1038/s41598-020-71329-8
Metabolic alterations in Parkinson's disease astrocytes
Abstract
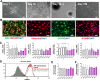
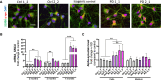
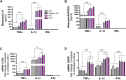
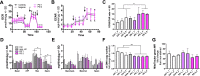
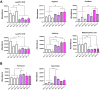
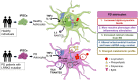
In Parkinson`s disease (PD), the loss of dopaminergic (DA) neurons in the substantia nigra pars compacta is associated with Lewy bodies arising from the accumulation of alpha-synuclein protein which leads ultimately to movement impairment. While PD has been considered a disease of the DA neurons, a glial contribution, in particular that of astrocytes, in PD pathogenesis is starting to be uncovered. Here, we report findings from astrocytes derived from induced pluripotent stem cells of LRRK2 G2019S mutant patients, with one patient also carrying a GBA N370S mutation, as well as healthy individuals. The PD patient astrocytes manifest the hallmarks of the disease pathology including increased expression of alpha-synuclein. This has detrimental consequences, resulting in altered metabolism, disturbed Ca2+ homeostasis and increased release of cytokines upon inflammatory stimulation. Furthermore, PD astroglial cells manifest increased levels of polyamines and polyamine precursors while lysophosphatidylethanolamine levels are decreased, both of these changes have been reported also in PD brain. Collectively, these data reveal an important role for astrocytes in PD pathology and highlight the potential of iPSC-derived cells in disease modeling and drug discovery.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Rocha EM, De Miranda B, Sanders LH. Alpha-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018;109:249–257. - PubMed
-
- Fymat, A. L. Parkinson’s Disease and other Movement Disorders: A Review. 28 (2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

