COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City
- PMID: 32879413
- PMCID: PMC7463226
- DOI: 10.1038/s41379-020-00661-1
COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City
Abstract
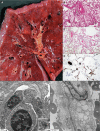
SARS-CoV-2, the etiologic agent of COVID-19, is a global pandemic with substantial mortality dominated by acute respiratory distress syndrome. We systematically evaluated lungs of 68 autopsies from 3 institutions in heavily hit areas (2 USA, 1 Italy). Detailed evaluation of several compartments (airways, alveolar walls, airspaces, and vasculature) was performed to determine the range of histologic features. The cohort consisted of 47 males and 21 females with a median age of 73 years (range 30-96). Co-morbidities were present in most patients with 60% reporting at least three conditions. Tracheobronchitis was frequently present, independent from intubation or superimposed pneumonia. Diffuse alveolar damage (DAD) was seen in 87% of cases. Later phases of DAD were less frequent and correlated with longer duration of disease. Large vessel thrombi were seen in 42% of cases but platelet (CD61 positive) and/or fibrin microthrombi were present at least focally in 84%. Ultrastructurally, small vessels showed basal membrane reduplication and significant endothelial swelling with cytoplasmic vacuolization. In a subset of cases, virus was detected using different tools (immunohistochemistry for SARS-CoV-2 viral spike protein, RNA in situ hybridization, lung viral culture, and electron microscopy). Virus was seen in airway epithelium and type 2 pneumocytes. IHC or in situ detection, as well as viable form (lung culture positive) was associated with the presence of hyaline membranes, usually within 2 weeks but up to 4 weeks after initial diagnosis. COVID-19 pneumonia is a heterogeneous disease (tracheobronchitis, DAD, and vascular injury), but with consistent features in three centers. The pulmonary vasculature, with capillary microthrombi and inflammation, as well as macrothrombi, is commonly involved. Viral infection in areas of ongoing active injury contributes to persistent and temporally heterogeneous lung damage.
Figures
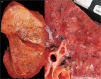
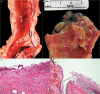

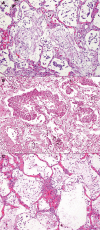


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

