Oestradiol as a neuromodulator of learning and memory
- PMID: 32879508
- PMCID: PMC8302223
- DOI: 10.1038/s41583-020-0362-7
Oestradiol as a neuromodulator of learning and memory
Abstract
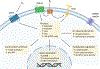
Although hormones such as glucocorticoids have been broadly accepted in recent decades as general neuromodulators of memory processes, sex steroid hormones such as the potent oestrogen 17β-oestradiol have been less well recognized by the scientific community in this capacity. The predominance of females in studies of oestradiol and memory and the general (but erroneous) perception that oestrogens are 'female' hormones have probably prevented oestradiol from being more widely considered as a key memory modulator in both sexes. Indeed, although considerable evidence supports a crucial role for oestradiol in regulating learning and memory in females, a growing body of literature indicates a similar role in males. This Review discusses the mechanisms of oestradiol signalling and provides an overview of the effects of oestradiol on spatial, object recognition, social and fear memories. Although the primary focus is on data collected in females, effects of oestradiol on memory in males will be discussed, as will sex differences in the molecular mechanisms that regulate oestrogenic modulation of memory, which may have important implications for the development of future cognitive therapeutics.
Conflict of interest statement
Competing interests
The authors declare the following competing interests: K.M.F. is a co-founder and the Chief Scientific Officer of Estrigenix Therapeutics, Inc, and is listed as an inventor of a pending patent held by the University of Wisconsin-Milwaukee, Marquette University, and Concordia University Wisconsin: ‘Substituted (4’-hydroxyphenyl)cycloalkane and (4’-hydroxyphenyl)cycloalkene compounds and uses thereof as selective agonists of the estrogen receptor beta isoform for enhanced memory consolidation’, inventors W. A. Donaldson, D. S. Sem & K.M.F.; WO2018183800A1. The other authors have no competing interests to declare.
Figures
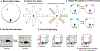

References
-
- Woolley CS & McEwen BS Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol 336, 293–306 (1993). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

