CO2 Capture by 2-(Methylamino)pyridine Ligated Aluminum Alkyl Complexes
- PMID: 32879618
- PMCID: PMC7457790
- DOI: 10.1002/ejic.202000437
CO2 Capture by 2-(Methylamino)pyridine Ligated Aluminum Alkyl Complexes
Abstract
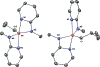
A set of novel, easily synthesized aluminum complexes, Al[κ2-N,N-2-(methylamino)pyridine]2R (R = Et, iBu) are reported. When subjected to 1 atm of CO2 pressure, each hemilabile pyridine arm dissociates and facilitates cooperative activation of the CO2 substrate reminiscent of a Frustrated Lewis Pair. This reaction has limited precedent for Al/N based Lewis Pair systems, and this is the first system readily shown to sequester multiple equivalents of CO2 per aluminum center. The ethyl variant then reacts further, inserting a third equivalent of CO2 into the aluminum alkyl to generate an aluminum carboxylate. Examples of this type of reactivity are rare under thermal conditions. A joint experimental/computational study supports the proposed reaction mechanism.
Keywords: Aluminum; Carbamate; Carbon dioxide; Frustrated Lewis pair; Insertion.
Figures


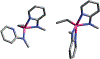
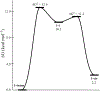
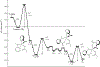


References
-
- Kohut R, Environment Int. 2003, 29, 171–180. - PubMed
-
- Mohtasham J, Energy Procedia 2015, 74, 1289–1297.
-
- Liu Y, Wang ZU, Zhaou HC, Greenh. Gases 2012, 2, 239–259.
-
- Pang S. Fuel Flexible Gas Production: Biomass, Coal, and Bio-Solid Wastes in Fuel Flexible Energy Generation (Ed.: Oakey J) Woodhead Publishing; 2016, pp. 241–269.
-
- Aresta M, Dibenedetto A, Dalton Trans. 2007, 2975–2992. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
