Photo-initiated rupture of azobenzene micelles to enable the spectroscopic analysis of antimicrobial peptide dynamics
- PMID: 32879729
- PMCID: PMC7449587
- DOI: 10.1039/d0ra01920h
Photo-initiated rupture of azobenzene micelles to enable the spectroscopic analysis of antimicrobial peptide dynamics
Abstract
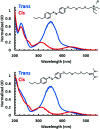
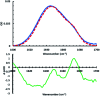
Antimicrobial peptides (AMPs) show promise for the treatment of bacterial infections, but many have undesired hemolytic activities. The AMP MP1 not only has broad spectrum bactericidal activity, but has been shown to have antitumor activity. The interaction between AMPs and cellular membranes gives rise to a peptide's cell-specificity and activity. However, direct analysis of the biophysical interactions between peptides and membrane is complex, in part due to the nature of membrane environments as well as structural changes in the peptide that occurs upon binding to the membrane. In order to investigate the interplay between cell selectivity, activity, and secondary structural changes involved in antimicrobial peptide activity, we sought to implement photolizable membrane mimics to assess the types of information available from infrared spectroscopic measurements that follow from photoinitiated peptide dynamics. Azo-surfactants (APEG) form micelles containing a photolizable azobenzene core, which upon irradiation can induce membrane deformation resulting in breakdown of micelles. Spectroscopic analysis of membrane deformation may provide insights into the physical behavior associated with unfolding and dissociation of antimicrobial peptides within a membrane environment. Herein, we synthesized and characterized two new azo-surfactants, APEGTMG and APEGNEt2MeI. Furthermore, we demonstrate the viability of azosurfactants as membrane mimics by examining both the membrane binding and dissociation induced secondary structural changes of the antimicrobial peptide, MP1.
This journal is © The Royal Society of Chemistry 2020.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Huang H. W. Biochemistry. 2000;39:8347–8352. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

