Co-Clinical Imaging Resource Program (CIRP): Bridging the Translational Divide to Advance Precision Medicine
- PMID: 32879897
- PMCID: PMC7442091
- DOI: 10.18383/j.tom.2020.00023
Co-Clinical Imaging Resource Program (CIRP): Bridging the Translational Divide to Advance Precision Medicine
Abstract
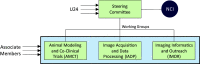
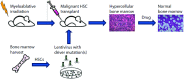
The National Institutes of Health's (National Cancer Institute) precision medicine initiative emphasizes the biological and molecular bases for cancer prevention and treatment. Importantly, it addresses the need for consistency in preclinical and clinical research. To overcome the translational gap in cancer treatment and prevention, the cancer research community has been transitioning toward using animal models that more fatefully recapitulate human tumor biology. There is a growing need to develop best practices in translational research, including imaging research, to better inform therapeutic choices and decision-making. Therefore, the National Cancer Institute has recently launched the Co-Clinical Imaging Research Resource Program (CIRP). Its overarching mission is to advance the practice of precision medicine by establishing consensus-based best practices for co-clinical imaging research by developing optimized state-of-the-art translational quantitative imaging methodologies to enable disease detection, risk stratification, and assessment/prediction of response to therapy. In this communication, we discuss our involvement in the CIRP, detailing key considerations including animal model selection, co-clinical study design, need for standardization of co-clinical instruments, and harmonization of preclinical and clinical quantitative imaging pipelines. An underlying emphasis in the program is to develop best practices toward reproducible, repeatable, and precise quantitative imaging biomarkers for use in translational cancer imaging and therapy. We will conclude with our thoughts on informatics needs to enable collaborative and open science research to advance precision medicine.
Keywords: CT; MR; cell transplant model (CTM); co-clinical trial; genetically engineered mouse model (GEMM); informatics; patient-derived tumor xenograft (PDX); precision medicine; preclinical PET; quantitative imaging.
© 2020 The Authors. Published by Grapho Publications, LLC.
Figures

References
-
- Chen Z, Akbay E, Mikse O, Tupper T, Cheng K, Wang Y, Tan X, Altabef A, Woo S-A, Chen L, Reibel JB, Janne PA, Sharpless NE, Engelman JA, Shapiro GI, Kung AL, Wong K-K. Co-clinical trials demonstrate superiority of crizotinib to chemotherapy in ALK-rearranged non-small cell lung cancer and predict strategies to overcome resistance. Clin Cancer Res. 2014;20:1204–1211. - PMC - PubMed
-
- Kim HR, Kang HN, Shim HS, Kim EY, Kim J, Kim DJ, Lee JG, Lee CY, Hong MH, Kim SM, Kim H, Pyo KH, Yun MR, Park HJ, Han JY, Youn HA, Ahn MJ, Paik S, Kim TM, Cho BC. Co-clinical trials demonstrate predictive biomarkers for dovitinib, an FGFR inhibitor, in lung squamous cell carcinoma. Ann Oncol. 2017;28:1250–1259. - PubMed
-
- Kwong LN, Boland GM, Frederick DT, Helms TL, Akid AT, Miller JP, Jiang S, Cooper ZA, Song X, Seth S, Kamara J, Protopopov A, Mills GB, Flaherty KT, Wargo JA, Chin L. Co-clinical assessment identifies patterns of BRAF inhibitor resistance in melanoma. J Clin Invest. 2015;125:1459–1470. - PMC - PubMed
-
- Lunardi A, Ala U, Epping MT, Salmena L, Clohessy JG, Webster KA, Wang G, Mazzucchelli R, Bianconi M, Stack EC, Lis R, Patnaik A, Cantley LC, Bubley G, Cordon-Cardo C, Gerald WL, Montironi R, Signoretti S, Loda M, Nardella C, Pandolfi PP. A co-clinical approach identifies mechanisms and potential therapies for androgen deprivation resistance in prostate cancer. Nat Genet. 2013;45:747–755. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

