Cost-effectiveness of Universal and Targeted Hepatitis C Virus Screening in the United States
- PMID: 32880650
- PMCID: PMC7489814
- DOI: 10.1001/jamanetworkopen.2020.15756
Cost-effectiveness of Universal and Targeted Hepatitis C Virus Screening in the United States
Abstract
Importance: Between 2 and 3.5 million people live with chronic hepatitis C virus (HCV) infection in the US, most of whom (approximately 75%) are not aware of their disease. Despite the availability of effective HCV treatment in the early stages of infection, HCV will result in thousands of deaths in the next decade in the US.
Objective: To investigate the cost-effectiveness of universal screening for all US adults aged 18 years or older for HCV in the US and of targeted screening of people who inject drugs.
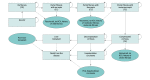
Design, setting, and participants: This simulated economic evaluation used cohort analyses in a Markov model to perform a 10 000-participant Monte Carlo microsimulation trail to evaluate the cost-effectiveness of HCV screening programs, and compared screening programs targeting people who inject drugs with universal screening of US adults age 18 years or older. Data were analyzed in December 2019.
Exposures: Cost per quality-adjusted life-year (QALY).
Main outcomes and measures: Cost per QALY gained.
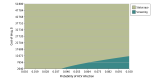
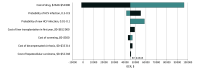
Results: In a 10 000 Monte Carlo microsimulation trail that compared a baseline of individuals aged 40 years (men and women) and people who inject drugs in the US, screening and treatment for HCV were estimated to increase total costs by $10 457 per person and increase QALYs by 0.23 (approximately 3 months), providing an incremental cost-effectiveness ratio of $45 465 per QALY. Also, universal screening and treatment for HCV are estimated to increase total costs by $2845 per person and increase QALYs by 0.01, providing an incremental cost-effectiveness ratio of $291 277 per QALY.
Conclusions and relevance: The findings of this study suggest that HCV screening for people who inject drugs may be a cost-effective intervention to combat HCV infection in the US, which could potentially decrease the risk of untreated HCV infection and liver-related mortality.
Conflict of interest statement
Figures
Comment in
-
Gaps in Descriptive Epidemiology and Hepatitis C Virus Modeling Research.JAMA Netw Open. 2020 Sep 1;3(9):e2016120. doi: 10.1001/jamanetworkopen.2020.16120. JAMA Netw Open. 2020. PMID: 32880647 No abstract available.
References
-
- Mitchell AE, Colvin HM. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. National Academies Press; 2010. - PubMed
-
- US Centers for Disease Control and Prevention Hepatits C. Published 2019. Accessed November 6, 2019. https://www.cdc.gov/hepatitis/hcv/index.htm

