Risk of chemotherapy-induced febrile neutropenia in patients with metastatic cancer not receiving granulocyte colony-stimulating factor prophylaxis in US clinical practice
- PMID: 32880732
- PMCID: PMC7892737
- DOI: 10.1007/s00520-020-05715-3
Risk of chemotherapy-induced febrile neutropenia in patients with metastatic cancer not receiving granulocyte colony-stimulating factor prophylaxis in US clinical practice
Abstract
Objectives: To evaluate the use of granulocyte colony-stimulating factor (G-CSF) prophylaxis in US patients with selected metastatic cancers and chemotherapy-induced febrile neutropenia (FN) incidence and associated outcomes among the subgroup who did not receive prophylaxis.
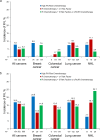
Methods: This retrospective cohort study was conducted at four US health systems and included adults with metastatic cancer (breast, colorectal, lung, non-Hodgkin lymphoma [NHL]) who received myelosuppressive chemotherapy (2009-2017). Patients were stratified by FN risk level based on risk factors and chemotherapy (low/unclassified risk, intermediate risk without any risk factors, intermediate risk with ≥ 1 risk factor [IR + 1], high risk [HR]). G-CSF use was evaluated among all patients stratified by FN risk, and FN/FN-related outcomes were evaluated among patients who did not receive first-cycle G-CSF prophylaxis.
Results: Among 1457 metastatic cancer patients, 20.5% and 28.1% were classified as HR and IR + 1, respectively. First-cycle G-CSF prophylaxis use was 48.5% among HR patients and 13.9% among IR + 1 patients. In the subgroup not receiving first-cycle G-CSF prophylaxis, FN incidence in cycle 1 was 7.8% for HR patients and 4.8% for IR + 1 patients; during the course, corresponding values were 16.9% and 15.9%. Most (> 90%) FN episodes required hospitalization, and mortality risk ranged from 7.1 to 26.9% across subgroups.
Conclusion: In this retrospective study, the majority of metastatic cancer chemotherapy patients for whom G-CSF prophylaxis is recommended did not receive it; FN incidence in this subgroup was notably high. Patients with elevated FN risk should be carefully identified and managed to ensure appropriate use of supportive care.
Keywords: Breast cancer; Colorectal cancer; Febrile neutropenia; Granulocyte colony-stimulating factor; Lung cancer; Non-Hodgkin lymphoma.
Conflict of interest statement
AA is employed by Policy Analysis Inc., which received funding for this research from Amgen Inc.
AS is employed by Policy Analysis Inc., which received funding for this research from Amgen Inc.
LL reports grants from Policy Analysis Inc.
KR-B is employed by Kaiser Permanente Northwest Region, Portland, OR, which received study funding from Amgen Inc.
MK has nothing to disclose.
DS has nothing to disclose.
NS is an employee of and owns stock in Amgen.
MH is an employee of and owns stock in Amgen.
TL is an employee of and owns stock in Amgen.
GHL reports serving as a consultant for Agendia, Amgen, Genomic Health, Halozyme Therapeutics, Mylan, Partners HealthCare, Pfizer, Samsung Bioepis, and Spectrum Pharmaceuticals.
DW is employed by Policy Analysis Inc., which received funding for this research from Amgen Inc.
Figures

References
-
- Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

