A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial
- PMID: 32880909
- PMCID: PMC8246960
- DOI: 10.1111/bjd.19509
A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial
Abstract
Background: Significantly more patients with moderate-to-severe plaque psoriasis treated with the interleukin (IL)-17A inhibitor ixekizumab vs. the IL-23p19 inhibitor guselkumab in the IXORA-R head-to-head trial achieved 100% improvement in Psoriasis Area and Severity Index (PASI 100) at week 12.
Objectives: To compare skin and nail clearance and patient-reported outcomes for ixekizumab vs. guselkumab, up to week 24.
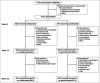
Methods: IXORA-R enrolled adults with moderate-to-severe plaque psoriasis, defined as static Physician's Global Assessment ≥ 3, PASI ≥ 12 and involved body surface area ≥ 10%. Statistical comparisons were performed using the Cochran-Mantel-Haenszel test stratified by pooled site. Time-to-first-event comparisons were performed using Kaplan-Meier analysis, and P-values were generated using adjusted log-rank tests stratified by treatment group. Cumulative days at clinical and patient-reported responses were compared by ancova. The trial was registered with ClinicalTrials.gov (NCT03573323).
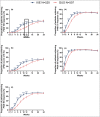
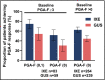
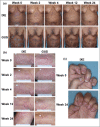
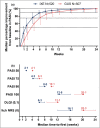
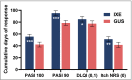
Results: Of the 1027 patients randomly assigned, 90% completed the trial (465 of 520 ixekizumab and 459 of 507 guselkumab). As early as week 2 and through week 16, more patients on ixekizumab achieved PASI 100 (P < 0·01). At week 24, ixekizumab was noninferior to guselkumab (50% vs. 52%, difference -2·3%), with no statistically significant difference in PASI 100 (P = 0·41). More patients receiving ixekizumab showed completely clear nails at week 24 (52% vs. 31%, P = 0·007). The median time to first PASI 50/75/90 and PASI 100 were 2 and 7·5 weeks shorter, respectively, for patients on ixekizumab vs. guselkumab (P < 0·001). Patients on ixekizumab also had a greater cumulative benefit, with more days at PASI 90 and 100, with Dermatology Life Quality Index of 0 or 1, and itch free (P < 0·05). The frequency of serious adverse events was 3% for each group, with no new safety signals.
Conclusions: Ixekizumab was noninferior to guselkumab in complete skin clearance and superior in clearing nails at week 24. Ixekizumab cleared skin more rapidly in patients with moderate-to-severe plaque psoriasis, with a greater cumulative benefit, than guselkumab. Overall, the safety findings were consistent with the known safety profile for ixekizumab.
© 2020 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures

Comment in
-
Head-to-head comparison of biologics in psoriasis: better acceleration does not necessarily win all races.Br J Dermatol. 2021 Jun;184(6):992-993. doi: 10.1111/bjd.19696. Epub 2020 Dec 21. Br J Dermatol. 2021. PMID: 33347616 No abstract available.
References
-
- Reich K, Griffiths CEM, Gordon KB et al. Maintenance of clinical response and consistent safety profile with up to 3 years of continuous treatment with guselkumab: results from the VOYAGE 1 and VOYAGE 2 trials. J Am Acad Dermatol 2020; 82:936–45. - PubMed
-
- Lebwohl MG, Bachelez H, Barker J et al. Patient perspectives in the management of psoriasis: results from the population‐based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol 2014; 70:871–81. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

