Submucosal enteric neurons of the cavine distal colon are sensitive to hypoosmolar stimuli
- PMID: 32880976
- PMCID: PMC7722197
- DOI: 10.1113/JP280309
Submucosal enteric neurons of the cavine distal colon are sensitive to hypoosmolar stimuli
Abstract
Key points: Neurons of the enteric submucous plexus are challenged by osmolar fluctuations during digestion and absorption of nutrients. Central neurons are very sensitive to changes in osmolality but knowledge on that issue related to enteric neurons is sparse. The present study focuses on investigation of osmosensitivity of submucosal neurons including potential molecular mediating mechanisms. Results show that submucosal neurons respond to hypoosmolar stimuli with increased activity which is partially mediated by the transient receptor potential vanilloid 4 channel. We provided important information on osmosensitive properties of enteric neurons. These data are fundamental to better explain the nerve-mediated control of the gastrointestinal functions during physiological and pathophysiological (diarrhoea) conditions.
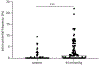
Abstract: Enteric neurons are located inside the gut wall, where they are confronted with changes in osmolality during (inter-) digestive periods. In particular, neurons of the submucous plexus (SMP), located between epithelial cells and blood vessels may sense and respond to osmotic shifts. The present study was conducted to investigate osmosensitivity of enteric submucosal neurons and the potential role of the transient receptor potential vanilloid 4 channel (TRPV4) as a mediator of enteric neuronal osmosensitivity. Therefore, freshly dissected submucosal preparations from guinea pig colon were investigated for osmosensitivity using voltage-sensitive dye and Ca2+ imaging. Acute hypoosmolar stimuli (final osmolality reached at ganglia of 94, 144 and 194 mOsm kg-1 ) were applied to single ganglia using a local perfusion system. Expression of TRPV4 in the SMP was quantified using qRT-PCR, and GSK1016790A and HC-067047 were used to activate or block the receptor, respectively, revealing its relevance in enteric osmosensitivity. On average, 11.0 [7.0/17.0] % of submucosal neurons per ganglion responded to the hypoosmolar stimulus. The Ca2+ imaging experiments showed that glia responded to the hypoosmolar stimulus, but with a delay in comparison with neurons. mRNA expression of TRPV4 could be shown in the SMP and blockade of the receptor by HC-067047 significantly decreased the number of responding neurons (0.0 [0.0/6.3] %) while the TRPV4 agonist GSK1016790A caused action potential discharge in a subpopulation of osmosensitive enteric neurons. The results of the present study provide insight into the osmosensitivity of submucosal enteric neurons and strongly indicate the involvement of TRPV4 as an osmotransducer.
Keywords: TRPV4; enteric nervous system; osmosensitivity; submucosal enteric neurons.
© 2020 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures


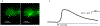


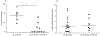

Comment in
-
Sensing osmolarity: a new player on the field.J Physiol. 2020 Dec;598(23):5297-5298. doi: 10.1113/JP280726. Epub 2020 Oct 2. J Physiol. 2020. PMID: 32965681 No abstract available.
Similar articles
-
Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity.J Neurosci. 1992 Jan;12(1):235-48. doi: 10.1523/JNEUROSCI.12-01-00235.1992. J Neurosci. 1992. PMID: 1729436 Free PMC article.
-
Chemical coding and electrophysiology of enteric neurons expressing neurofilament 145 in guinea pig gastrointestinal tract.J Comp Neurol. 2002 Jan 14;442(3):189-203. J Comp Neurol. 2002. PMID: 11774335
-
Immunohistochemical localisation of cholinergic muscarinic receptor subtype 1 (M1r) in the guinea pig and human enteric nervous system.J Chem Neuroanat. 2007 Jul;33(4):193-201. doi: 10.1016/j.jchemneu.2007.03.001. Epub 2007 Mar 23. J Chem Neuroanat. 2007. PMID: 17462859
-
Submucosal secretomotor and vasodilator reflexes.Neurogastroenterol Motil. 2004 Apr;16 Suppl 1:39-43. doi: 10.1111/j.1743-3150.2004.00473.x. Neurogastroenterol Motil. 2004. PMID: 15066003 Review.
-
The Role of the TRPV4 Channel in Intestinal Physiology and Pathology.J Inflamm Res. 2024 Nov 21;17:9307-9317. doi: 10.2147/JIR.S483350. eCollection 2024. J Inflamm Res. 2024. PMID: 39588136 Free PMC article. Review.
Cited by
-
Piezo2 regulates colonic mechanical sensitivity in a sex specific manner in mice.Nat Commun. 2023 Apr 15;14(1):2158. doi: 10.1038/s41467-023-37683-7. Nat Commun. 2023. PMID: 37061508 Free PMC article.
-
TRPV4 is expressed by enteric glia and muscularis macrophages of the colon but does not play a prominent role in colonic motility.bioRxiv [Preprint]. 2024 Jan 11:2024.01.09.574831. doi: 10.1101/2024.01.09.574831. bioRxiv. 2024. PMID: 38260314 Free PMC article. Preprint.
-
Neurochemical Aspects of the Role of Thirst in Body Fluid Homeostasis and Their Significance in Health and Disease: A Literature Review.Int J Mol Sci. 2025 Aug 14;26(16):7850. doi: 10.3390/ijms26167850. Int J Mol Sci. 2025. PMID: 40869169 Free PMC article. Review.
-
Current Practice in Using Voltage Imaging to Record Fast Neuronal Activity: Successful Examples from Invertebrate to Mammalian Studies.Biosensors (Basel). 2023 Jun 13;13(6):648. doi: 10.3390/bios13060648. Biosensors (Basel). 2023. PMID: 37367013 Free PMC article. Review.
References
-
- Adachi A. (1984). Thermosensitive and osmoreceptive afferent fibers in the hepatic branch of the vagus nerve. J Auton Nerv Syst 10, 269–273. - PubMed
-
- Baertschi AJ & Pence RA. (1995). Gut-brain signaling of water absorption inhibits vasopressin in rats. Am J Physiol 268, R236–247. - PubMed
-
- Baylis PH. (1983). Posterior pituitary function in health and disease. Clin Endocrinol Metab 12, 747–770. - PubMed
-
- Bertrand PP, Kunze WA, Bornstein JC, Furness JB & Smith ML. (1997). Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am J Physiol 273, G422–435. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

