Hydroxychloroquine and Chloroquine Prescribing Patterns by Provider Specialty Following Initial Reports of Potential Benefit for COVID-19 Treatment - United States, January-June 2020
- PMID: 32881845
- PMCID: PMC7470458
- DOI: 10.15585/mmwr.mm6935a4
Hydroxychloroquine and Chloroquine Prescribing Patterns by Provider Specialty Following Initial Reports of Potential Benefit for COVID-19 Treatment - United States, January-June 2020
Abstract
Hydroxychloroquine and chloroquine, primarily used to treat autoimmune diseases and to prevent and treat malaria, received national attention in early March 2020, as potential treatment and prophylaxis for coronavirus disease 2019 (COVID-19) (1). On March 20, the Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for chloroquine phosphate and hydroxychloroquine sulfate in the Strategic National Stockpile to be used by licensed health care providers to treat patients hospitalized with COVID-19 when the providers determine the potential benefit outweighs the potential risk to the patient.* Following reports of cardiac and other adverse events in patients receiving hydroxychloroquine for COVID-19 (2), on April 24, 2020, FDA issued a caution against its use† and on June 15, rescinded its EUA for hydroxychloroquine from the Strategic National Stockpile.§ Following the FDA's issuance of caution and EUA rescindment, on May 12 and June 16, the federal COVID-19 Treatment Guidelines Panel issued recommendations against the use of hydroxychloroquine or chloroquine to treat COVID-19; the panel also noted that at that time no medication could be recommended for COVID-19 pre- or postexposure prophylaxis outside the setting of a clinical trial (3). However, public discussion concerning the effectiveness of these drugs on outcomes of COVID-19 (4,5), and clinical trials of hydroxychloroquine for prophylaxis of COVID-19 continue.¶ In response to recent reports of notable increases in prescriptions for hydroxychloroquine or chloroquine (6), CDC analyzed outpatient retail pharmacy transaction data to identify potential differences in prescriptions dispensed by provider type during January-June 2020 compared with the same period in 2019. Before 2020, primary care providers and specialists who routinely prescribed hydroxychloroquine, such as rheumatologists and dermatologists, accounted for approximately 97% of new prescriptions. New prescriptions by specialists who did not typically prescribe these medications (defined as specialties accounting for ≤2% of new prescriptions before 2020) increased from 1,143 prescriptions in February 2020 to 75,569 in March 2020, an 80-fold increase from March 2019. Although dispensing trends are returning to prepandemic levels, continued adherence to current clinical guidelines for the indicated use of these medications will ensure their availability and benefit to patients for whom their use is indicated (3,4), because current data on treatment and pre- or postexposure prophylaxis for COVID-19 indicate that the potential benefits of these drugs do not appear to outweigh their risks.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
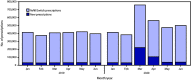
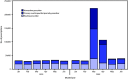
References
-
- National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. Rockville, MD: US Department of Health and Human Services, National Institutes of Health; 2020. https://www.covid19treatmentguidelines.nih.gov/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

