Seroprevalence of SARS-CoV-2 Among Frontline Health Care Personnel in a Multistate Hospital Network - 13 Academic Medical Centers, April-June 2020
- PMID: 32881855
- PMCID: PMC7470460
- DOI: 10.15585/mmwr.mm6935e2
Seroprevalence of SARS-CoV-2 Among Frontline Health Care Personnel in a Multistate Hospital Network - 13 Academic Medical Centers, April-June 2020
Abstract
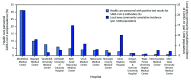
Health care personnel (HCP) caring for patients with coronavirus disease 2019 (COVID-19) might be at high risk for contracting SARS-CoV-2, the virus that causes COVID-19. Understanding the prevalence of and factors associated with SARS-CoV-2 infection among frontline HCP who care for COVID-19 patients are important for protecting both HCP and their patients. During April 3-June 19, 2020, serum specimens were collected from a convenience sample of frontline HCP who worked with COVID-19 patients at 13 geographically diverse academic medical centers in the United States, and specimens were tested for antibodies to SARS-CoV-2. Participants were asked about potential symptoms of COVID-19 experienced since February 1, 2020, previous testing for acute SARS-CoV-2 infection, and their use of personal protective equipment (PPE) in the past week. Among 3,248 participants, 194 (6.0%) had positive test results for SARS-CoV-2 antibodies. Seroprevalence by hospital ranged from 0.8% to 31.2% (median = 3.6%). Among the 194 seropositive participants, 56 (29%) reported no symptoms since February 1, 2020, 86 (44%) did not believe that they previously had COVID-19, and 133 (69%) did not report a previous COVID-19 diagnosis. Seroprevalence was lower among personnel who reported always wearing a face covering (defined in this study as a surgical mask, N95 respirator, or powered air purifying respirator [PAPR]) while caring for patients (5.6%), compared with that among those who did not (9.0%) (p = 0.012). Consistent with persons in the general population with SARS-CoV-2 infection, many frontline HCP with SARS-CoV-2 infection might be asymptomatic or minimally symptomatic during infection, and infection might be unrecognized. Enhanced screening, including frequent testing of frontline HCP, and universal use of face coverings in hospitals are two strategies that could reduce SARS-CoV-2 transmission.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Christopher J. Lindsell reports grants from National Institutes of Health, the Department of Defense, and the Marcus Foundation, and contract support from Endpoint Health, Entegrion, bioMerieux, and Bioscape Digital, outside the submitted work. Daniel J. Henning reports personal fees from CytoVale and grants from Baxter, outside the submitted work. Akram Khan reports grants from United Therapeutics, Actelion Pharmaceuticals, Regeneron, and Reata Pharmaceuticals, outside the submitted work. Samuel M. Brown reports grants from National Institutes of Health, Department of Defense, Intermountain Research and Medical Foundation, and Janssen; consulting fees paid to his employer from Faron and Sedana, all outside the submitted work. Ithan D. Peltan reports grants from the National Institutes of Health and, outside the submitted work, grants from Asahi Kasei Pharma, Immunexpress Inc., Janssen Pharmaceuticals, and Regeneron. Carlos G. Grijalva reports personal fees from Pfizer, Merck, and Sanofi-Pasteur, grants from Campbell Alliance, the National Institutes of Health, the Food and Drug Administration, and the Agency for Health Care Research and Quality, outside the submitted work. Todd W. Rice reports consulting work for Cumberland Pharmaceuticals, Inc., Cytovale, Inc., and Avisa, LLC, outside the submitted work. H. Keipp Talbot has served on a data safety and monitoring board for Seqirus. No other potential conflicts of interest were disclosed.
Figures
References
-
- Stubblefield WB, Talbot HK, Feldstein L, et al. ; Influenza Vaccine Effectiveness in the Critically Ill (IVY) Investigators. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for COVID-19 patients—Nashville, Tennessee. Clin Infect Dis 2020; Epub July 6, 2020. 10.1093/cid/ciaa936 - DOI - PMC - PubMed
-
- US Department of Homeland Security. Advisory memorandum on identification of essential critical infrastructure workers during COVID-19 response. Washington, DC: US Department of Homeland Security; 2020. https://www.cisa.gov/sites/default/files/publications/Version_3.0_CISA_G...

