Rejection of intestinal allotransplants is driven by memory T helper type 17 immunity and responds to infliximab
- PMID: 32882110
- PMCID: PMC8049508
- DOI: 10.1111/ajt.16283
Rejection of intestinal allotransplants is driven by memory T helper type 17 immunity and responds to infliximab
Abstract
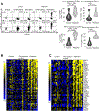
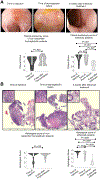
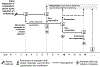
Intestinal transplantation (ITx) can be life-saving for patients with advanced intestinal failure experiencing complications of parenteral nutrition. New surgical techniques and conventional immunosuppression have enabled some success, but outcomes post-ITx remain disappointing. Refractory cellular immune responses, immunosuppression-linked infections, and posttransplant malignancies have precluded widespread ITx application. To shed light on the dynamics of ITx allograft rejection and treatment resistance, peripheral blood samples and intestinal allograft biopsies from 51 ITx patients with severe rejection, alongside 37 stable controls, were analyzed using immunohistochemistry, polychromatic flow cytometry, and reverse transcription-PCR. Our findings inform both immunomonitoring and treatment. In terms of immunomonitoring, we found that while ITx rejection is associated with proinflammatory and activated effector memory T cells in the blood, evidence of treatment efficacy can only be found in the allograft itself, meaning that blood-based monitoring may be insufficient. In terms of treatment, we found that the prominence of intra-graft memory TNF-α and IL-17 double-positive T helper type 17 (Th17) cells is a leading feature of refractory rejection. Anti-TNF-α therapies appear to provide novel and safer treatment strategies for refractory ITx rejection; with responses in 14 of 14 patients. Clinical protocols targeting TNF-α, IL-17, and Th17 warrant further testing.
Keywords: T cell biology; clinical research/practice; immunobiology; immunosuppression/immune modulation; immunosuppressive regimens; intestinal (allograft) function/dysfunction; intestine/multivisceral transplantation; mucosal immunity; rejection; translational research/science.
© 2020 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures




Similar articles
-
Mucosal plasma cell barrier disruption during intestine transplant rejection.Transplantation. 2012 Dec 27;94(12):1236-42. doi: 10.1097/TP.0b013e3182708d9d. Transplantation. 2012. PMID: 23269450
-
Characterization of T cell immunophenotypes in intestinal transplantation: A pilot study.Transpl Immunol. 2018 Dec;51:50-57. doi: 10.1016/j.trim.2018.09.003. Epub 2018 Sep 20. Transpl Immunol. 2018. PMID: 30243797 Free PMC article.
-
Complete freedom from rejection after intestinal transplantation using a new tolerogenic protocol combined with low immunosuppression.Transplantation. 2002 Mar 27;73(6):966-8. doi: 10.1097/00007890-200203270-00024. Transplantation. 2002. PMID: 11923701
-
Intestinal Transplant Immunology and Intestinal Graft Rejection: From Basic Mechanisms to Potential Biomarkers.Int J Mol Sci. 2023 Feb 25;24(5):4541. doi: 10.3390/ijms24054541. Int J Mol Sci. 2023. PMID: 36901975 Free PMC article. Review.
-
Present status and future perspectives of intestinal transplantation.Transpl Int. 2008 May;21(5):401-14. doi: 10.1111/j.1432-2277.2008.00637.x. Epub 2008 Feb 18. Transpl Int. 2008. PMID: 18282247 Review.
Cited by
-
Immune cell profiling in intestinal transplantation.Hum Immunol. 2024 Jul;85(4):110808. doi: 10.1016/j.humimm.2024.110808. Epub 2024 May 18. Hum Immunol. 2024. PMID: 38762429 Free PMC article.
-
The Microbiome and Metabolomic Profile of the Transplanted Intestine with Long-Term Function.Biomedicines. 2022 Aug 25;10(9):2079. doi: 10.3390/biomedicines10092079. Biomedicines. 2022. PMID: 36140180 Free PMC article.
-
Low regulatory T-cells frequency is associated with graft rejection after small bowel transplantation: Clinical and experimental evidence.PLoS One. 2025 Jan 24;20(1):e0307534. doi: 10.1371/journal.pone.0307534. eCollection 2025. PLoS One. 2025. PMID: 39854413 Free PMC article.
-
Regulatory T Cell and T Helper 17 Cell Imbalance in Patients with Unexplained Infertility.Int J Womens Health. 2024 May 31;16:1033-1040. doi: 10.2147/IJWH.S455733. eCollection 2024. Int J Womens Health. 2024. PMID: 38835834 Free PMC article.
-
CD69+ resident memory T cells are associated with graft-versus-host disease in intestinal transplantation.Am J Transplant. 2021 May;21(5):1878-1892. doi: 10.1111/ajt.16405. Epub 2021 Feb 17. Am J Transplant. 2021. PMID: 33226726 Free PMC article.
References
-
- Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 2003;124(4):1111–1134. - PubMed
-
- Beath S, Pironi L, Gabe S, Horslen S, Sudan D, Mazeriegos G et al. Collaborative strategies to reduce mortality and morbidity in patients with chronic intestinal failure including those who are referred for small bowel transplantation. Transplantation 2008;85(10):1378–1384. - PubMed
-
- Fishbein TM, Matsumoto CS. Intestinal replacement therapy: timing and indications for referral of patients to an intestinal rehabilitation and transplant program. Gastroenterology 2006;130(2 Suppl 1):S147–151. - PubMed
-
- Fishbein TM. Intestinal transplantation. N Engl J Med 2009;361(10):998–1008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

