Evaluation of SARS-CoV-2 in Tears of Patients with Moderate to Severe COVID-19
- PMID: 32882309
- PMCID: PMC7458068
- DOI: 10.1016/j.ophtha.2020.08.029
Evaluation of SARS-CoV-2 in Tears of Patients with Moderate to Severe COVID-19
Abstract
Purpose: To investigate the presence of SARS-CoV-2 RNA in tears of patients with moderate to severe coronavirus disease 2019 (COVID-19).
Design: Cross-sectional study.
Participants: Patients with laboratory-proven moderate to severe COVID-19.
Methods: Tears were collected within 48 hours of laboratory confirmation using 3 methods: conjunctival swab plus Schirmer's test strips (group 1), conjunctival swab (group 2), and Schirmer's test strips (group 3). Samples from both the eyes of each patient were transported in a single viral transport media for real-time RT-PCR. Detailed demographic profiles, systemic symptoms, comorbidities, and ocular manifestations were noted.
Main outcome measures: Viral load of a sample was determined using cycle threshold (Ct) value of E gene. A specimen was considered to show positive results if the amplification curve for the E gene crossed the threshold line within 35 cycles and if it showed positive results on an RNA-dependent RNA polymerase or open reading frame 1b gene assay.
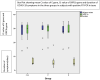
Results: Of the 78 patients enrolled in the study, samples from 3 patients were found to be inadequate for analysis. Thirty-six patients (48%) had moderate disease, whereas 39 patients (52%) had severe disease, with no ocular involvement in any patient. In the 75 patients, RT-PCR analysis of tears showed positive results in 18 patients (24%), and 29 of 225 samples (12.9%) showed positive results. Positive results were found in 11 (14.7%), 11 (14.7%), and 7 (9.3%) patients in groups 1, 2, and 3, respectively (P = 0.3105). Mean Ct values in groups 1, 2, and 3 were 28.36 ± 6.15, 29.00 ± 5.58, and 27.86 ± 6.46 (P = 0.92), respectively. Five patients showed positive RT-PCR results by all 3 methods (mean Ct value, 25.24 ± 6.33), and 12 patients showed positive results by any of the 3 methods (mean Ct value, 32.16 ± 1.94), the difference in Ct values being statistically significant (P = 0.029). The median value of symptomatology in patients with positive RT-PCR results from tears was 5 days (range, 4-9 days).
Conclusions: SARS-CoV-2 RNA was detected in tears of 24% of patients with laboratory-proven moderate to severe COVID-19. Conjunctival swab remains the gold standard of tear collection for RT-PCR assay. A significantly higher possibility of viral transmission exists through tears in patients with moderate to severe COVID-19.
Keywords: COVID-19; Conjunctival swab; Nasopharyngeal swab; Reverse-transcriptase polymerase chain reaction; SARS-CoV-2.
Copyright © 2020 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Commentary: SARS-CoV-2 in tears: It really matters.Indian J Ophthalmol. 2021 Dec;69(12):3636-3637. doi: 10.4103/ijo.IJO_2391_21. Indian J Ophthalmol. 2021. PMID: 34827011 Free PMC article. No abstract available.
References
-
- Woo P.C., Lau S.K., Huang Y., Yuen K.Y. Coronavirus diversity, phylogeny and interspecies jumping. Exp Biol Med. 2009;234(10):1117–1127. - PubMed
-
- Lan J., Ge J., Yu J., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

