SARS-CoV-2 antibodies, serum inflammatory biomarkers and clinical severity of hospitalized COVID-19 patients
- PMID: 32882666
- PMCID: PMC7459327
- DOI: 10.1016/j.jcv.2020.104611
SARS-CoV-2 antibodies, serum inflammatory biomarkers and clinical severity of hospitalized COVID-19 patients
Abstract
Background: The involvement of SARS-CoV-2 antibodies in mediating immunopathogenetic events in COVID-19 patients has been suggested. By using several experimental approaches, we investigated the potential association between SARS-CoV-2 IgGs recognizing the spike (S) protein receptor-binding domain (RBD), neutralizing antibodies (NtAb) targeting S, and COVID-19 severity.
Patients and methods: This unicenter, retrospective, observational study included 51 hospitalized patients (24 at the intensive care unit; ICU). A total of 93 sera from these patients collected at different time points from the onset of symptoms were analyzed. SARS-CoV-2 RBD IgGs were quantitated by ELISA and NtAb50 titers were measured in a GFP reporterbased pseudotyped virus platform. Demographic and clinical data, complete blood counts, as well as serum levels of ferritin, Dimer-D, C reactive protein (CRP), lactose dehydrogenase (LDH), and interleukin-6 (IL-6) were retrieved from clinical charts.
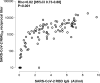
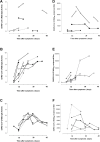
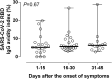
Results: The overall correlation between levels of both antibody measurements was good (Rho = 0.82; P = 0 < 0.001). SARS-CoV-2 RBD IgG and NtAb50 levels in sera collected up to day 30 after the onset of symptoms were comparable between ICU and non-ICU patients (P=>0.1). Four ICU patients died; two of these achieved NtAb50 titers ≥1/160 while the other two exhibited a 1/80 titer. Very weak (Rho=>0.0-<0.2) or weak (Rho=>0.2-<0.4) correlations were observed between anti-RBD IgGs, NtAb50, and serum levels pro-inflammatory biomarkers.
Conclusions: The data presented herein do not support an association between SARS-CoV-2 RBD IgG or NtAb50 levels and COVID-19 severity.
Keywords: COVID-19; Inflammatory biomarkers; Neutralizing antibodies; SARS-CoV-2.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors report no declarations of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

