ApoE Lipidation as a Therapeutic Target in Alzheimer's Disease
- PMID: 32882843
- PMCID: PMC7503657
- DOI: 10.3390/ijms21176336
ApoE Lipidation as a Therapeutic Target in Alzheimer's Disease
Abstract
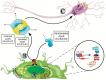
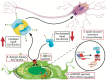
Apolipoprotein E (APOE) is the major cholesterol carrier in the brain, affecting various normal cellular processes including neuronal growth, repair and remodeling of membranes, synaptogenesis, clearance and degradation of amyloid β (Aβ) and neuroinflammation. In humans, the APOE gene has three common allelic variants, termed E2, E3, and E4. APOE4 is considered the strongest genetic risk factor for Alzheimer's disease (AD), whereas APOE2 is neuroprotective. To perform its normal functions, apoE must be secreted and properly lipidated, a process influenced by the structural differences associated with apoE isoforms. Here we highlight the importance of lipidated apoE as well as the APOE-lipidation targeted therapeutic approaches that have the potential to correct or prevent neurodegeneration. Many of these approaches have been validated using diverse cellular and animal models. Overall, there is great potential to improve the lipidated state of apoE with the goal of ameliorating APOE-associated central nervous system impairments.
Keywords: apolipoprotein E; cholesterol; lipid homeostasis; neurodegeneration.
Conflict of interest statement
The authors declare no conflict of interest. G.W.R. is a member of the APOE Scientific Advisory Board at Biogen.
Figures
References
-
- Strittmatter W.J., Saunders A.M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G.S., Roses A.D. Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. - DOI - PMC - PubMed
-
- Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.W., Roses A.D., Haines J.L., Pericak-Vance M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

