ATP Production Relies on Fatty Acid Oxidation Rather than Glycolysis in Pancreatic Ductal Adenocarcinoma
- PMID: 32882923
- PMCID: PMC7564784
- DOI: 10.3390/cancers12092477
ATP Production Relies on Fatty Acid Oxidation Rather than Glycolysis in Pancreatic Ductal Adenocarcinoma
Abstract
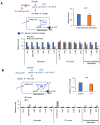
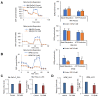
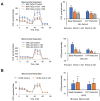
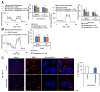
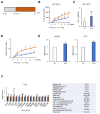
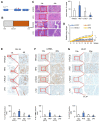
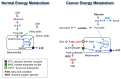
Glycolysis is known as the main pathway for ATP production in cancer cells. However, in cancer cells, glucose deprivation for 24 h does not reduce ATP levels, whereas it does suppress lactate production. In this study, metabolic pathways were blocked to identify the main pathway of ATP production in pancreatic ductal adenocarcinoma (PDAC). Blocking fatty acid oxidation (FAO) decreased ATP production by 40% in cancer cells with no effect on normal cells. The effects of calorie balanced high- or low-fat diets were tested to determine whether cancer growth is modulated by fatty acids instead of calories. A low-fat diet caused a 70% decrease in pancreatic preneoplastic lesions compared with the control, whereas a high-fat diet caused a two-fold increase in preneoplastic lesions accompanied with increase of ATP production in the Kras (G12D)/Pdx1-cre PDAC model. The present results suggest that ATP production in cancer cells is dependent on FAO rather than on glycolysis, which can be a therapeutic approach by targeting cancer energy metabolism.
Keywords: ATP production; KC mouse; PDAC; fatty acid oxidation; glycolysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. - PubMed
-
- Xu R.H., Pelicano H., Zhou Y., Carew J.S., Feng L., Bhalla K.N., Keating M.J., Huang P. Inhibition of glycolysis in cancer cells: A novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65:613–621. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

