Efficacy of Heterologous Prime-Boost Vaccination with H3N2 Influenza Viruses in Pre-Immune Individuals: Studies in the Pig Model
- PMID: 32882956
- PMCID: PMC7552030
- DOI: 10.3390/v12090968
Efficacy of Heterologous Prime-Boost Vaccination with H3N2 Influenza Viruses in Pre-Immune Individuals: Studies in the Pig Model
Abstract
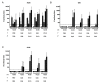
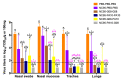
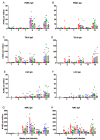
In a previous study in influenza-naïve pigs, heterologous prime-boost vaccination with monovalent, adjuvanted whole inactivated vaccines (WIV) based on the European swine influenza A virus (SwIAV) strain, A/swine/Gent/172/2008 (G08), followed by the US SwIAV strain, A/swine/Pennsylvania/A01076777/2010 (PA10), was shown to induce broadly cross-reactive hemagglutination inhibition (HI) antibodies against 12 out of 15 antigenically distinct H3N2 influenza strains. Here, we used the pig model to examine the efficacy of that particular heterologous prime-boost vaccination regimen, in individuals with pre-existing infection-immunity. Pigs were first inoculated intranasally with the human H3N2 strain, A/Nanchang/933/1995. Seven weeks later, they were vaccinated intramuscularly with G08 followed by PA10 or vice versa. We examined serum antibody responses against the hemagglutinin and neuraminidase, and antibody-secreting cell (ASC) responses in peripheral blood, draining lymph nodes, and nasal mucosa (NMC), in ELISPOT assays. Vaccination induced up to 10-fold higher HI antibody titers than in naïve pigs, with broader cross-reactivity, and protection against challenge with an antigenically distant H3N2 strain. It also boosted ASC responses in lymph nodes and NMC. Our results show that intramuscular administration of WIV can lead to enhanced antibody responses and cross-reactivity in pre-immune subjects, and recall of ASC responses in lymph nodes and NMC.
Keywords: H3N2; antibody cross-reactivity; heterologous prime-boost; humans; influenza; pre-existing immunity; swine; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Van Reeth K., Vincent A. Influenza viruses. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J., Stevenson G.W., Zhang J., editors. Diseases of Swine. 11th ed. John Wiley & Sons, Incorporated; Hoboken, NJ, USA: 2019. pp. 576–593.
-
- Nelson M.I., Wentworth D.E., Culhane M.R., Vincent A.L., Viboud C., LaPointe M.P., Lin X., Holmes E.C., Detmer S.E. Introductions and evolution of human-origin seasonal influenza a viruses in multinational swine populations. J. Virol. 2014;88:10110–10119. doi: 10.1128/JVI.01080-14. - DOI - PMC - PubMed
-
- Haesebrouck F., Biront P., Pensaert M.B., Leunen J. Epizootics of respiratory tract disease in swine in Belgium due to H3N2 influenza virus and experimental reproduction of disease. Am. J. Vet. Res. 1985;46:1926–1928. - PubMed
-
- de Jong J.C., Smith D.J., Lapedes A.S., Donatelli I., Campitelli L., Barigazzi G., Van Reeth K., Jones T.C., Rimmelzwaan G.F., Osterhaus A.D., et al. Antigenic and genetic evolution of swine influenza A (H3N2) viruses in Europe. J. Virol. 2007;81:4315–4322. doi: 10.1128/JVI.02458-06. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

