Potential of Ocular Transmission of SARS-CoV-2: A Review
- PMID: 32883010
- PMCID: PMC7559808
- DOI: 10.3390/vision4030040
Potential of Ocular Transmission of SARS-CoV-2: A Review
Abstract
Purpose of review: to provide a prospective on the current mechanisms by which SARS-CoV-2 enters cells and replicates, and its implications for ocular transmission. The literature was analyzed to understand ocular transmission as well as molecular mechanisms by which SARS-CoV-2 enters cells and replicates. Analysis of gene expression profiles from available datasets, published immunohistochemistry, as well as current literature was reviewed, to assess the likelihood that ocular inoculation of SARS-CoV-2 results in systemic infection.
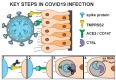
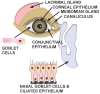
Recent findings: The ocular surface and retina have the necessary proteins, Transmembrane Serine Protease 2 (TMPRSS2), CD147, Angiotensin-Converting Enzyme 2 (ACE2) and Cathepsin L (CTSL) necessary to be infected with SARS-CoV-2. In addition to direct ocular infection, virus carried by tears through the nasolacrimal duct to nasal epithelium represent a means of ocular inoculation.
Summary: There is evidence that SARS-CoV-2 may either directly infect cells on the ocular surface, or virus can be carried by tears through the nasolacrimal duct to infect the nasal or gastrointestinal epithelium.
Keywords: COVID-19; SARS-CoV-2; conjunctivitis; coronavirus; ocular transmission.
Conflict of interest statement
The authors do not have any relevant conflicts of interest.
Figures




References
-
- Raboud J., Shigayeva A., McGeer A., Bontovics E., Chapman M., Gravel D., Henry B., Lapinsky S., Loeb M., McDonald L.C., et al. Risk factors for SARS transmission from patients requiring intubation: A multicentre investigation in Toronto, Canada. PLoS ONE. 2010;5:e10717. doi: 10.1371/journal.pone.0010717. - DOI - PMC - PubMed
-
- Sungnak W., Huang N., Becavin C., Berg M., Queen R., Litvinukova M., Talavera-Lopez C., Maatz H., Reichart D., Sampaziotis F., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

