Positive association between physical outcomes and patient-reported outcomes in late-onset Pompe disease: a cross sectional study
- PMID: 32883321
- PMCID: PMC7469279
- DOI: 10.1186/s13023-020-01469-7
Positive association between physical outcomes and patient-reported outcomes in late-onset Pompe disease: a cross sectional study
Abstract
Background: Pompe disease is a rare, progressive metabolic myopathy. The aim of this study is to investigate the associations of physical outcomes with patient-reported outcome measures (PROMs) in late-onset Pompe disease.
Methods: We included 121 Dutch adult patients with Pompe disease. Physical outcomes comprised muscle strength (manual muscle testing using Medical Research Council [MRC] grading, hand-held dynamometry [HHD]), walking ability (6-min walk test [6MWT]), and pulmonary function (forced vital capacity [FVC] in upright and supine positions). PROMs comprised quality of life (Short Form 36 health survey [SF-36]), participation (Rotterdam Handicap Scale [RHS]) and daily-life activities (Rasch-Built Pompe-Specific Activity [R-PAct] Scale). Analyses were cross-sectional: the time-point before, and closest to, start of Enzyme Replacement Therapy was chosen. Associations between PROMs and physical outcomes were investigated using linear regression models.
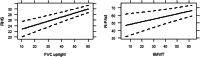
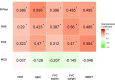
Results: RHS and R-PAct scores were better in patients with higher FVC supine and upright, HHD, MRC and 6MWT scores, accounting for the effect of sex, disease duration, use of wheelchair and ventilator support. While the SF-36 Physical Component Summary (PCS) was correlated positively with FVC upright, HHD, MRC and 6MWT scores, there was no significant relationship between the SF-36 Mental Component Summary (MCS) and any of the physical outcomes.
Conclusions: Participation, daily-life activities, and the physical component of quality of life of adult Pompe patients are positively correlated to physical outcomes. This work serves as a first step towards assessing how changes over time in physical outcomes are related to changes in PROMs, and to define the minimal change in physical outcomes required to make an important difference for the patient.
Keywords: 6-min walk test; Forced vital capacity; Late-onset Pompe disease; Muscle strength; Patient-reported outcome measures.
Conflict of interest statement
A. van der Ploeg received funding for research, clinical trials and as an advisor from various industries working on ERT or next-generation therapies in the field of Pompe disease, other lysosomal storage diseases, and neuromuscular disorders under agreements with Erasmus MC University Medical Center and the relevant industry. N. van der Beek received funding for research and as advisor from various industries working on ERT or next-generation therapies in the field of Pompe disease under agreements with Erasmus MC University Medical Center and the relevant industry. The other authors declare that they have no conflict of interest.
Figures
Similar articles
-
Switching treatment to cipaglucosidase alfa plus miglustat positively affects patient-reported outcome measures in patients with late-onset Pompe disease.J Patient Rep Outcomes. 2024 Nov 13;8(1):132. doi: 10.1186/s41687-024-00805-w. J Patient Rep Outcomes. 2024. PMID: 39535661 Free PMC article. Clinical Trial.
-
Association between changes in pulmonary function and in patient reported outcomes during enzyme therapy of adult patients with late-onset Pompe disease.J Inherit Metab Dis. 2023 Jul;46(4):595-604. doi: 10.1002/jimd.12606. Epub 2023 Apr 2. J Inherit Metab Dis. 2023. PMID: 36966448
-
Long-term benefit of enzyme replacement therapy in Pompe disease: A 5-year prospective study.Neurology. 2017 Dec 5;89(23):2365-2373. doi: 10.1212/WNL.0000000000004711. Epub 2017 Nov 8. Neurology. 2017. PMID: 29117951
-
Forced vital capacity and cross-domain late-onset Pompe disease outcomes: an individual patient-level data meta-analysis.J Neurol. 2019 Sep;266(9):2312-2321. doi: 10.1007/s00415-019-09401-1. Epub 2019 Jun 11. J Neurol. 2019. PMID: 31187190 Free PMC article.
-
The clinical relevance of outcomes used in late-onset Pompe disease: can we do better?Orphanet J Rare Dis. 2013 Oct 12;8:160. doi: 10.1186/1750-1172-8-160. Orphanet J Rare Dis. 2013. PMID: 24119230 Free PMC article. Review.
Cited by
-
Quality of Life with Late-Onset Pompe Disease: Qualitative Interviews and General Public Utility Estimation in the United Kingdom.J Health Econ Outcomes Res. 2023 Mar 3;10(1):41-50. doi: 10.36469/001c.68157. eCollection 2023. J Health Econ Outcomes Res. 2023. PMID: 36883056 Free PMC article.
-
The impact of COVID-19 infection, the pandemic and its associated control measures on patients with Pompe disease.J Neurol. 2024 Jan;271(1):32-45. doi: 10.1007/s00415-023-11999-2. Epub 2023 Nov 20. J Neurol. 2024. PMID: 37982853 Free PMC article.
-
Glycogen storage diseases.Nat Rev Dis Primers. 2023 Sep 7;9(1):46. doi: 10.1038/s41572-023-00456-z. Nat Rev Dis Primers. 2023. PMID: 37679331 Review.
-
Application of Patient-Reported Outcome Measurements in Clinical Trials in China.JAMA Netw Open. 2022 May 2;5(5):e2211644. doi: 10.1001/jamanetworkopen.2022.11644. JAMA Netw Open. 2022. PMID: 35544134 Free PMC article.
-
Switching treatment to cipaglucosidase alfa plus miglustat positively affects patient-reported outcome measures in patients with late-onset Pompe disease.J Patient Rep Outcomes. 2024 Nov 13;8(1):132. doi: 10.1186/s41687-024-00805-w. J Patient Rep Outcomes. 2024. PMID: 39535661 Free PMC article. Clinical Trial.
References
-
- Hirschhorn R, Reuser AJ, et al. Glycogen storage disease type II: acid alpha-glucosidase (acid maltase) deficiency. In: Scriver CR, Beaudet AL, Valle D, Sly W, Childs B, Kinzler KW, et al., editors. The metabolic and molecular bases of inherited disease. 8. New York: McGraw-Hill; 2001. pp. 3389–3420.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

