SARS-CoV-2 serology and virology trends in donors and recipients of convalescent plasma
- PMID: 32883593
- PMCID: PMC7446657
- DOI: 10.1016/j.transci.2020.102922
SARS-CoV-2 serology and virology trends in donors and recipients of convalescent plasma
Abstract
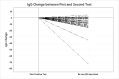
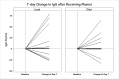
SARS-CoV-2 has infected millions worldwide. The virus is novel, and currently there is no approved treatment. Convalescent plasma may offer a treatment option. We evaluated trends of IgM/IgG antibodies/plasma viral load in donors and recipients of convalescent plasma. 114/139 (82 %) donors had positive IgG antibodies. 46/114 donors tested positive a second time by NP swab. Among those retested, the median IgG declined (p < 0.01) between tests. 25/139 donors with confirmed SARS-CoV-2 were negative for IgG antibodies. This suggests that having had the infection does not necessarily convey immunity, or there is a short duration of immunity associated with a decline in antibodies. Plasma viral load obtained on 35/39 plasma recipients showed 22 (62.9 %) had non-detectable levels on average 14.5 days from positive test versus 6.2 days in those with detectable levels (p < 0.01). There was a relationship between IgG and viral load. IgG was higher in those with non-detectable viral loads. There was no relationship between viral load and blood type (p = 0.87) or death (0.80). Recipients with detectable viral load had lower IgG levels; there was no relationship between viral load, blood type or death.
Keywords: Adults; Antigens; Blood transfusion; COVID-19; COVID-19 serotherapy; Coronavirus; Coronavirus infections; Humans; Immunoglobulin G; Immunoglobulin M; Plasma; SARS virus; Severe acute respiratory syndrome; Severe acute respiratory syndrome coronavirus 2; Viral; Viral load.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report – 66. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... 2020 March 26 (cited 2020 15 June).
-
- Johns Hopkins University and Medicine. Coronavirus resource center. COVID-19 global cases (cited 2020 Aug 6). https://coronavirus.jhu.edu/map.html.
-
- US Food and Drug Administration . 2020. Investigational COVID-19 convalescent plasma: emergency INDs.https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-in... (accessed 2020 March 30; guidance updated 2020 May 1)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous