A Comprehensive Gender-related Secretome of Plasmodium berghei Sexual Stages
- PMID: 32883804
- PMCID: PMC7710150
- DOI: 10.1074/mcp.RA120.002212
A Comprehensive Gender-related Secretome of Plasmodium berghei Sexual Stages
Abstract
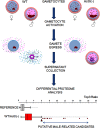
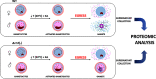
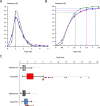
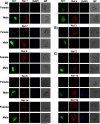
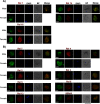
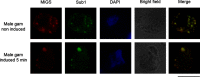
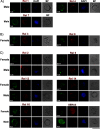
Plasmodium, the malaria parasite, undergoes a complex life cycle alternating between a vertebrate host and a mosquito vector of the genus Anopheles In red blood cells of the vertebrate host, Plasmodium multiplies asexually or differentiates into gamete precursors, the male and female gametocytes, responsible for parasite transmission. Sexual stage maturation occurs in the midgut of the mosquito vector, where male and female gametes egress from the host erythrocytes to fuse and form a zygote. Gamete egress entails the successive rupture of two membranes surrounding the parasite, the parasitophorous vacuole membrane and the erythrocyte plasma membrane. In this study, we used the rodent model parasite Plasmodium berghei to design a label-free quantitative proteomic approach aimed at identifying gender-related proteins differentially released/secreted by purified mature gametocytes when activated to form gametes. We compared the abundance of molecules secreted by wild type gametocytes of both genders with that of a transgenic line defective in male gamete maturation and egress. This enabled us to provide a comprehensive data set of egress-related molecules and their gender specificity. Using specific antibodies, we validated eleven candidate molecules, predicted as either gender-specific or common to both male and female gametocytes. All of them localize to punctuate, vesicle-like structures that relocate to cell periphery upon activation, but only three of them localize to the gametocyte-specific secretory vesicles named osmiophilic bodies. Our results confirm that the egress process involves a tightly coordinated secretory apparatus that includes different types of vesicles and may put the basis for functional studies aimed at designing novel transmission-blocking molecules.
Keywords: Parasite; Plasmodium berghei; blood; egress; gametocyte; infectious disease; label-free quantification; malaria; mass spectrometry; osmiophilic bodies; pathogens; secretome.
© 2020 Grasso et al.
Conflict of interest statement
Conflict of interest—Authors declare no competing interests.
Figures
References
-
- Schneider P., Bousema J. T., Gouagna L. C., Otieno S., van de Vegte-Bolmer M., Omar S. A., and Sauerwein R. W. (2007) Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am. J. Trop. Med. Hyg. 76, 470–474 - PubMed
-
- Garcia G. E., Wirtz R. A., Barr J. R., Woolfitt A., and Rosenberg R. (1998) Xanthurenic acid induces gametogenesis in Plasmodium, the malaria parasite. J. Biol. Chem. 273, 12003–12005 - PubMed
-
- Sologub L., Kuehn A., Kern S., Przyborski J., Schillig R., and Pradel G. (2011) Malaria proteases mediate inside-out egress of gametocytes from red blood cells following parasite transmission to the mosquito. Cell Microbiol. 13, 897–912 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

