Cell-free DNA concentration in patients with clinical or mammographic suspicion of breast cancer
- PMID: 32884019
- PMCID: PMC7471679
- DOI: 10.1038/s41598-020-71357-4
Cell-free DNA concentration in patients with clinical or mammographic suspicion of breast cancer
Abstract
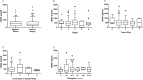
Mammography has a crucial role in the detection of breast cancer (BC), yet it is not limitation-free. We hypothesized that the combination of mammography and cell-free DNA (cfDNA) levels may better discriminate patients with cancer. This prospective study included 259 participants suspected with BC before biopsy. Blood samples were taken before biopsy and from some patients during and at the end of treatment. cfDNA blood levels were measured using our simple fluorescent assay. The primary outcome was the pathologic diagnosis of BC, and the secondary aims were to correlate cfDNA to severity, response to treatments, and outcome. Median cfDNA blood levels were similar in patients with positive and negative biopsy: 577 vs. 564 ng/ml (p = 0.98). A significant decrease in cfDNA blood level was noted after the following treatments: surgery, surgery and radiation, neo-adjuvant chemotherapy and surgery, and at the end of all treatments. To conclude, the cfDNA level could not be used in suspected patients to discriminate BC. Reduction of tumor burden by surgery and chemotherapy is associated with reduction of cfDNA levels. In a minority of patients, an increase in post-treatment cfDNA blood level may indicate the presence of a residual tumor and higher risk. Further outcome assessment for a longer period is suggested.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Wiratkapun C, Treesit T, Wibulpolprasert B, Lertsithichai P. Diagnostic accuracy of ultrasonography-guided core needle biopsy for breast lesions. Singapore Med. J. 2012;53:40–45. - PubMed
-
- Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646–650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

