Evaluating the Therapeutic Potential of Cenicriviroc in the Treatment of Nonalcoholic Steatohepatitis with Fibrosis: A Brief Report on Emerging Data
- PMID: 32884369
- PMCID: PMC7434517
- DOI: 10.2147/HMER.S230613
Evaluating the Therapeutic Potential of Cenicriviroc in the Treatment of Nonalcoholic Steatohepatitis with Fibrosis: A Brief Report on Emerging Data
Abstract
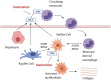
Non-alcoholic steatohepatitis (NASH) is associated with significant morbidity and mortality due to liver cirrhosis, liver failure, and hepatocellular carcinoma, and represents a leading indication for liver transplantation in the United States (U.S.). A growing spectrum of novel therapies are currently in clinical development and target several mechanisms of action which address hepatic steatosis, steatohepatitis, and hepatic fibrosis. Cenicriviroc (Allergan, Dublin, Ireland) is a novel oral antagonist of CC-motif chemokine receptors 2 and 5 (CCR2/5) which have demonstrated expression on circulating monocytes and Kupffer cells. Preclinical models have confirmed that CCR2/5 antagonism may block fat accumulation and Kupffer cell activation and disrupt monocyte/macrophage recruitment and hepatic stellate cell activation responsible for fibrogenesis. Herein we review results from the phase 2b CENTAUR trial and study designs for the phase 2b TANDEM and phase 3 AURORA trials and discuss the potential role of cenicriviroc in future pharmacotherapy for NASH fibrosis.
Keywords: antifibrotic therapy; cenicriviroc; clinical trials; fatty liver; liver fibrosis; non-alcoholic steatohepatitis.
© 2020 Diaz Soto and Lim.
Conflict of interest statement
JKL reports research contracts (to Yale University) from Allergan, Conatus, Genfit, Gilead, and Intercept. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Cenicriviroc for the treatment of non-alcoholic steatohepatitis and liver fibrosis.Expert Opin Investig Drugs. 2018 Mar;27(3):301-311. doi: 10.1080/13543784.2018.1442436. Epub 2018 Feb 22. Expert Opin Investig Drugs. 2018. PMID: 29448843 Review.
-
Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis.Hepatology. 2018 Apr;67(4):1270-1283. doi: 10.1002/hep.29544. Epub 2018 Feb 19. Hepatology. 2018. PMID: 28940700
-
Antifibrotic Effects of the Dual CCR2/CCR5 Antagonist Cenicriviroc in Animal Models of Liver and Kidney Fibrosis.PLoS One. 2016 Jun 27;11(6):e0158156. doi: 10.1371/journal.pone.0158156. eCollection 2016. PLoS One. 2016. PMID: 27347680 Free PMC article.
-
Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design.Contemp Clin Trials. 2016 Mar;47:356-65. doi: 10.1016/j.cct.2016.02.012. Epub 2016 Mar 2. Contemp Clin Trials. 2016. PMID: 26944023 Clinical Trial.
-
Future Pharmacotherapy for Non-alcoholic Steatohepatitis (NASH): Review of Phase 2 and 3 Trials.J Clin Transl Hepatol. 2018 Sep 28;6(3):264-275. doi: 10.14218/JCTH.2017.00056. Epub 2018 Jun 28. J Clin Transl Hepatol. 2018. PMID: 30271738 Free PMC article. Review.
Cited by
-
Design and Synthesis of Fluorescence-Labeled TAK779 Analogs as Chemical Probes.Molecules. 2025 Jun 19;30(12):2655. doi: 10.3390/molecules30122655. Molecules. 2025. PMID: 40572618 Free PMC article.
-
LILRB2/PirB mediates macrophage recruitment in fibrogenesis of nonalcoholic steatohepatitis.Nat Commun. 2023 Jul 22;14(1):4436. doi: 10.1038/s41467-023-40183-3. Nat Commun. 2023. PMID: 37481670 Free PMC article.
-
Treatment of Fatty Liver Disease: The Present and the Future.Cureus. 2021 Jan 15;13(1):e12713. doi: 10.7759/cureus.12713. Cureus. 2021. PMID: 33614318 Free PMC article. Review.
-
Alcohol-associated liver disease: Natural history, management and novel targeted therapies.Clin Mol Hepatol. 2025 Feb;31(Suppl):S112-S133. doi: 10.3350/cmh.2024.0709. Epub 2024 Oct 31. Clin Mol Hepatol. 2025. PMID: 39481875 Free PMC article. Review.
-
On the Role of Paraoxonase-1 and Chemokine Ligand 2 (C-C motif) in Metabolic Alterations Linked to Inflammation and Disease. A 2021 Update.Biomolecules. 2021 Jul 1;11(7):971. doi: 10.3390/biom11070971. Biomolecules. 2021. PMID: 34356595 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources